This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 03:03, 12 July 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 03:03, 12 July 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChemSpiderID_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation ()(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)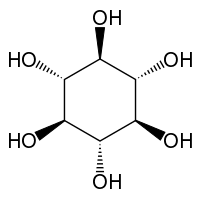
| |
| Names | |
|---|---|
| IUPAC name (1R,2R,3R,4R,5R,6R)-Cyclohexane-1,2,3,4,5,6-hexaol | |
| Other names Scyllitol; Cocositol; Quercinitol; AZD 103; 1,3,5/2,4,6-Hexahydroxycyclohexane; scyllo-Cyclohexanehexol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.113.358 |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C6H12O6 |
| Molar mass | 180.156 g·mol |
| Appearance | White crystalline solid |
| Melting point | 348.5-350 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
scyllo-Inositol is one of the stereoisomers of inositol. It is also known as scyllitol, cocositol, quercinitol, and 1,3,5/2,4,6-hexahydroxycyclohexane. scyllo-Inositol is a naturally occurring plant sugar alcohol found most abundantly in the coconut palm.
Biological effects
Researchers at the University of Toronto have found that scyllo-inositol can block the development of amyloid-beta (Aβ) plaques in the brains of transgenic mice. scyllo-Inositol also reversed memory deficits, reduced the formation of Aβ plaques, and alleviated other symptoms that are associated with the accumulation of Aβ proteins in these mice.
Clinical evaluation
scyllo-Inositol is under investigation by Transition Therapeutics as a disease-modifying therapy for Alzheimer's disease under the designation AZD-103. A patent was issued on April 21, 2009 (US patent number 7,521,481) claiming the use of scyllo-inositol for treating Alzheimer's disease. scyllo-Inositol is undergoing clinical investigation as an orally-administered therapeutic agent for the treatment of mild to moderate Alzheimer's disease. It has received fast track designation from the U.S. Food and Drug Administration. Transition has partnered with Elan Corporation on the development of the compound under the designation ELND005. ELND005 is currently in a Phase 2 clinical study, which completed enrollment in October 2008. The study is a randomized, double-blind, placebo-controlled, dose-ranging, safety and efficacy study in approximately 353 patients with mild to moderate Alzheimer's disease. The planned treatment period for each patient is approximately 18 months.
In December 2009, Elan and Transition jointly reported that the study has been modified so that only the 250 mg twice daily dose will be continued because of greater rates of adverse events, including 9 deaths, in the higher dose groups (1000 mg and 2000 mg dosed twice daily).
External Links
Drugs In Clinical Trials - ELND005
References
- Scyllitol, Dr. Duke's Phytochemical and Ethnobotanical Databases
- Aβ Busters and Other Ploys Show Promise for Treating Neurodegeneration
- "Cyclohexanehexol inhibitors of Abeta aggregation prevent and reverse Alzheimer phenotype in a mouse model". 12 (7). 2006: 801–8. doi:10.1038/nm1423. PMID 16767098.
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|month=ignored (help) - Elan and Transition Therapeutics Receive Key Patent for Alzheimer's Disease Treatment with ELND005
- Elan and Transition Therapeutics Announce Modifications to ELND005 Phase II Clinical Trials in Alzheimer's Disease