This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 15:09, 7 August 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per [[Misplaced Pages:WikiProject Chemicals/Chembox validation|Chem/). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 15:09, 7 August 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per [[Misplaced Pages:WikiProject Chemicals/Chembox validation|Chem/)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Chloroacetaldehyde | |||
| Systematic IUPAC name Chloroethanal | |||
| Identifiers | |||
| CAS Number | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.158 | ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
| Properties | |||
| Chemical formula | C2H3Cl | ||
| Molar mass | 78.50 g mol | ||
| Appearance | Colourless liquid | ||
| Boiling point | 85–85.5 °C | ||
| Solubility in water | Soluble as hydrate | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | alkylating agent | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
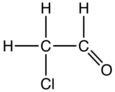
Chloroacetaldehyde is the organic compound with the formula ClCH2CHO. Like some related compounds, it is highly electrophilic reagent and a potentially dangerous alkylating agent. The compound is not normally encountered in the anhydrous form, but rather as the hydrate (acetal), ClCH2CH(OH)2. Chloroacetaldehyde is a useful intermediate in the synthesis, e.g. of 2-aminothiazole or many pharmaceutical compounds. Another use is to facilitate bark removal from tree trunks.
Synthesis and reactions
The hydrate of chloroacetaldehyde is produced by the oxidation of aqueous vinyl chloride using chlorine:
- ClCH=CH2 + Cl2 + H2O → ClCH2CHO + 2 HCl
It can also be prepared from vinyl acetate.
Being bifunctional, chloroacetaldehyde is a versatile precursor to many heterocyclic compounds. It condenses with thiourea derivatives to give aminothiazoles. This reaction was once important as a precursor to sulfathiazole, one of the first sulfa drugs.
Environmental aspects
Chloroacetaldehyde is a metabolite in the degradation of 1,2-dichloroethane, which initially converts to chloroethanol. This metabolic pathway is topical since billions of kilograms of 1,2-dichloroethane have been produced as a precursor to vinyl chloride.
References
- ^ Reinhard Jira, Erwin Kopp, Blaine C. McKusick, Gerhard Röderer, Axel Bosch, Gerald Fleischmann “Chloroacetaldehydes“ in Ullmann's Encyclopedia of Industrial Chemistry, 2007, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_527.pub2
- Janssen, D. B.; van der Ploeg, J. R. and Pries, F., "Genetics and biochemistry of 1,2-dichloroethane degradation", Biodegradation, 1994, 5, 249-57.doi:10.1007/BF00696463