This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 17:52, 9 August 2011 (Updating {{drugbox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Misplaced Pages talk:WikiProject_P). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 17:52, 9 August 2011 by CheMoBot (talk | contribs) (Updating {{drugbox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Misplaced Pages talk:WikiProject_P)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound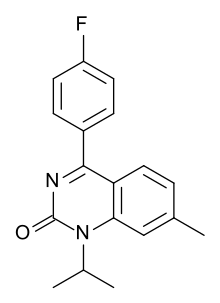 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H17FN2O |
| Molar mass | 296.339 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Fluproquazone (trade name Tormosyl) is a quinazolinone derivative with potent analgesic and antipyretic effects and also anti-inflammatory action. It has been shown to be effective in a variety of animal species after both oral and parenteral administration, and has a duration of action of several hours. The compound is many times more potent than acetylsalicylic acid and clinically generally resembles ibuprofen and indoprofen in its pharmacological effects, but with significantly less ulcerogenic activity. It is mainly used in the treatment of arthritis and post-operative pain.
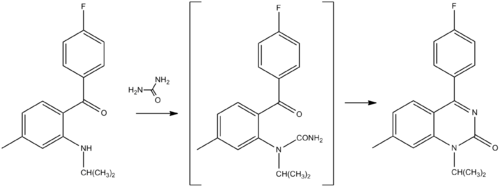
Chemistry
Lindler, J.; Mattner, P. G.; Salmond, W. G.; 1973, U.S. patent 3,759,920.
References
- Mohing W, Suckert R, Lataste X. Comparative study of fluproquazone in the management of post-operative pain. Arzneimittelforschung. 1981;31(5a):918-20.
- Wheatley D. Analgesic properties of fluproquazone. Rheumatology and Rehabilitation. 1982 May;21(2):98-100.
- Fankhauser S, Laube W, Marti HR, Schultheiss HR, Vogtlin J, von Graffenried B. Antipyretic activity of fluproquazone in man. Arzneimittelforschung. 1981;31(5a):934-5.
- Gillberg R, Korsan-Bengtsen K, Magnusson B, Nyberg G. Gastrointestinal blood loss, gastroscopy and coagulation factors in normal volunteers during administration of acetylsalicylic acid and fluproquazone. Scandinavian Journal of Rheumatology. 1981;10(4):342-6.
- Huskisson EC, Bryans R, Scott J. Fluproquazone for osteoarthritis. Rheumatology and Rehabilitation. 1981 May;20(2):122-4.
- Haanaes HR, Benterud UJ, Skoglund LA. RF 46-790 versus paracetamol: effect on post-operative pain. International Journal of Clinical Pharmacology, Therapy, and Toxicology. 1986 Nov;24(11):598-601.
| Non-steroidal anti-inflammatory drugs (NSAIDs) (primarily M01A and M02A, also N02BA) | |
|---|---|
| pyrazolones / pyrazolidines | |
| salicylates | |
| acetic acid derivatives and related substances | |
| oxicams | |
| propionic acid derivatives (profens) |
|
| n-arylanthranilic acids (fenamates) | |
| COX-2 inhibitors (coxibs) | |
| other | |
| NSAID combinations | |
| Key: underline indicates initially developed first-in-class compound of specific group; WHO-Essential Medicines; withdrawn drugs; veterinary use. | |