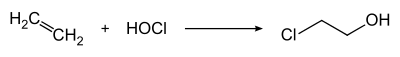This is an old revision of this page, as edited by Vchorozopoulos (talk | contribs) at 09:38, 10 August 2011. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 09:38, 10 August 2011 by Vchorozopoulos (talk | contribs)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
| |

| |
| Names | |
|---|---|
| IUPAC name 2-Chloroethanol | |
| Other names 2-chloroethyl alcohol, ethylene chlorohydrin, glycol chlorohydrin, 2-chloro-1-ethanol, 2-monochloroethanol, 2-hydroxyethyl chloride, β-chloroethanol, β-hydroxyethyl chloride, chloroethanol, δ-chloroethanol, ethylchlorhydrin, ethylene chlorohydrin, glycol monochlorohydrin | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.146 |
| KEGG | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C2H5ClO |
| Molar mass | 80.52 g/mol |
| Density | 1.197 g/cm³ |
| Melting point | -67 °C |
| Boiling point | 128-130 °C |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
2-Chloroethanol is an organochlorine compound with the formula HOCH2CH2Cl. This colorless liquid has a pleasant ether-like odor. It is miscible with water. The molecule is bifunctional, consisting of both an alkyl chloride and an alcohol functional groups.
Synthesis and applications
2-Chloroethanol is produced by treating ethylene with hypochlorous acid:
2-Chloroethanol was once produced on a large scale as a precursor to ethylene oxide:
This application has been supplanted by the greener direct oxidation of ethylene. Otherwise chloroethanol is used in a number of specialized applications. Several dyes are prepared by the alkylation of aniline derivatives with chloroethanol. It is a building block in the production of pharmaceuticals, biocides and plasticizers. It is also used for manufacture of thiodiglycol. It is a solvent for cellulose acetate and ethyl cellulose, textile printing dyes, in dewaxing, refining of rosin, extraction of pine lignin, and the cleaning of machines.
Environmental aspects
Chloroethanol is a metabolite in the degradation of 1,2-dichloroethane. The alcohol is then further oxidized via chloroacetaldehyde to chloroacetate. This metabolic pathway is topical since billions of kilograms of 1,2-dichloroethane are processed annually as a precursor to vinyl chloride.
Safety
2-Chloroethanol is toxic with an LD50 of 89 mg/kg in rats. Like most organochlorine compounds, chloroethanol combusts to yield hydrogen chloride and phosgene.
References
- Gordon Y. T. Liu, W. Frank Richey, Joanne E. Betso “Chlorohydrins“ in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_565
- Roderich Raue and John F. Corbett “Nitro and Nitroso Dyes“ in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_383
- 1. Janssen, D. B.; van der Ploeg, J. R. and Pries, F., "Genetics and Biochemistry of 1,2-Dichloroethane Degradation", Biodegradation, 1994, volume 5, pp. 249-57.doi:10.1007/BF00696463