This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 20:02, 14 August 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemicals|error). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 20:02, 14 August 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemicals|error)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)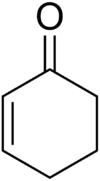
| |
| Names | |
|---|---|
| IUPAC name 1-Cyclohex-2-enone | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.012.021 |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H8O |
| Molar mass | 96.12712 |
| Appearance | Clear colorless liquid |
| Density | 0.993 g/mL |
| Melting point | −53 °C |
| Boiling point | 171-173 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Cyclohexenone is an organic compound which is a versatile intermediate used in the synthesis of a variety of chemical products such as pharmaceuticals and fragrances. It is a clear colorless liquid with a boiling point of 171-173 °C.
Industrially, cyclohexenone is prepared from phenol by Birch reduction.
Common reactions involving cyclohexenone include nucleophilic conjugate addition with organocopper reagents, Michael reactions and Robinson annulations.
References
- Cyclohexenone at Sigma-Aldrich
- Podraze, K.F. Org. Prep. Proced. Int., 1991, 23, p. 217.
- Organic Building Blocks of the Chemical Industry, Harry H. Szmant, ISBN 978-0471855453
- Michael G. Organ and Paul Anderson (1996). "Carbonyl and Conjugate Additions to Cyclohexenone: Experiments Illustrating Reagent Selectivity". Journal of Chemical Education. 73 (12): 1193. doi:10.1021/ed073p1193.
- Tet. Lett. 34, 3881, (1993)