This is an old revision of this page, as edited by Atomician (talk | contribs) at 15:26, 21 August 2011 (→Acetalisation of carbonyl groups by alcohols). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 15:26, 21 August 2011 by Atomician (talk | contribs) (→Acetalisation of carbonyl groups by alcohols)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| It has been suggested that this article be merged into acetal. (Discuss) Proposed since February 2011. |
Acetalisation is an organic reaction that involves the formation of an acetal or ketal. One way of acetal formation is the nucleophilic addition of an alcohol to a ketone or an aldehyde. Acetalisation is often used in organic synthesis to create a protecting group because it is a reversible reaction.
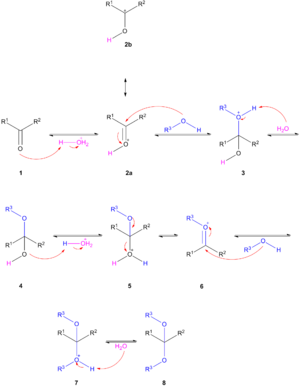
Acetalisation is acid catalysed with elimination of water. The reaction can be driven to the acetal when water is removed from the reaction system either by azeotropic distillation or trapping water with molecular sieves or aluminium oxide. The general reaction mechanism for acetalisation of a carbonyl group is shown to the right.
The carbonyl group in 1 abstracts a proton from hydrochloric acid. The protonated carbonyl group 2 is activated for nucleophilic addition of the alcohol. The structures 2a and 2b are mesomers. After deprotonation of 3 by water the hemiacetal or hemiketal 4 is formed. The hydroxyl group in 4 is protonated leading to the oxonium ion 6 which accepts a second alcohol group to 7 with a final deprotonation to the acetal 8. The reverse reaction takes place by adding water in the same acidic medium. Acetals are stable towards basic media. In a transacetalisation or crossacetalisation a diol reacts with an acetal or two different acetals react with each other. Again this is possible because all the reaction steps are equilibria.