This is an old revision of this page, as edited by Lamro (talk | contribs) at 14:43, 30 August 2011 (link). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 14:43, 30 August 2011 by Lamro (talk | contribs) (link)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)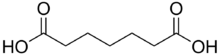
| |

| |
| Names | |
|---|---|
| IUPAC name heptanedioic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.003.492 |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C7H12O4 |
| Molar mass | 160.17 g/mol |
| Density | 1.28 g/cm |
| Melting point | 103-105 °C |
| Boiling point | Decomposes |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Pimelic acid is the organic compound with the formula HO2C(CH2)5CO2H. Derivatives of pimelic acid are involved in the biosynthesis of the amino acid called lysine. Pimelic acid is one methylene longer than a related dicarboxylic acid, adipic acid, a precursor to many polyesters and polyamides. It is the final member of the mnemonic used to aid recollection of the order of the first six dicarboxylic acids using their common (not IUPAC) nomenclature: Dicarboxylic acid
Pimelic acid has been synthesized from cyclohexanone and from salicylic acid. In the former route, the additional carbon is supplied by dimethyloxalate, which reacts with the enolate.
See also
References
- Snyder, H. R.; Brooks, A. L.; Shapiro, S. H. "Pimelic Acid from Cyclohexanone" and Müller, A. "Pimelic Acid from Salicylic Acid" Organic Syntheses, Collected Volume 2, p.531 (1943).http://www.orgsyn.org/orgsyn/pdfs/CV2P0531.pdf