This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 21:36, 31 August 2011 (Updating {{drugbox}} (no changed fields - added verified revid - updated 'ChemSpiderID_Ref', 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per [[WP:CHEMVALID|Chem/Drugbox validation). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 21:36, 31 August 2011 by CheMoBot (talk | contribs) (Updating {{drugbox}} (no changed fields - added verified revid - updated 'ChemSpiderID_Ref', 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per [[WP:CHEMVALID|Chem/Drugbox validation)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound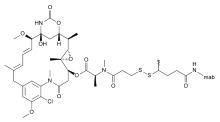 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Target | CD44 v6 |
| Clinical data | |
| ATC code |
|
| (verify) | |
Bivatuzumab mertansine is a combination of bivatuzumab, a humanized monoclonal antibody, and mertansine, a cytotoxic agent. It is designed for the treatment of squamous cell carcinoma.
References
- Tijink, BM; Buter, J; De Bree, R; Giaccone, G; Lang, MS; Staab, A; Leemans, CR; Van Dongen, GA (2006). "A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus". Clinical cancer research : an official journal of the American Association for Cancer Research. 12 (20 Pt 1): 6064–72. doi:10.1158/1078-0432.CCR-06-0910. PMID 17062682.
This monoclonal antibody–related article is a stub. You can help Misplaced Pages by expanding it. |