This is an old revision of this page, as edited by CheMoBot (talk | contribs) at 03:12, 16 September 2011 (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or [[user talk:CheMoBo). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 03:12, 16 September 2011 by CheMoBot (talk | contribs) (Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or [[user talk:CheMoBo)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Hydrogen deuteride" – news · newspapers · books · scholar · JSTOR (December 2008) (Learn how and when to remove this message) |
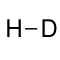
| |
| Names | |
|---|---|
| IUPAC name Hydrogen deuteride | |
| Systematic IUPAC name (H)Dihydrogen | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.034.325 |
| EC Number |
|
| PubChem CID | |
| UN number | 1049 |
| CompTox Dashboard (EPA) | |
InChI
| |
|
SMILES
| |
| Properties | |
| Chemical formula | H |
| Molar mass | 3.02204 g mol |
| Melting point | −259 °C (−434.2 °F; 14.1 K) |
| Boiling point | −253 °C (−423.4 °F; 20.1 K) |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Hydrogen deuteride is a diatomic molecule composed of the two isotopes of hydrogen: the majority isotope H protium and H deuterium. Its molecular formula is HD.
Availability in nature
Hydrogen deuteride is a minor component of naturally occurring molecular hydrogen. However, Hydrogen deuteride does not chemically behave exactly the same has H2—and can thus serve as an important marker.
In particular Hydrogen deuteride is one of the minor but noticeable components of the atmospheres of all the giant planets, with abundances from about 30 ppm to about 200 ppm. HD has also been found in Supernova remnants, and other sources.
Gas Giants occourance (HD vs H2)
- Jupiter : ~0.003% :: 89.8% ±2.0%
- Uranus : ~0.007% :: 83.0% ±3.0%
- Neptune : ~0.019% :: 80.0% ±3.2%
Radio emission spectra
HD and H2 do have very similar emission spectra, but don't emit on exactly the same frequencies .
The frequency of the astronomically important J = 1-0 rotational transition of HD at 2.7 THz has been measured with tunable FIR radiation with an accuracy of 150 kHz .
References
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |