This is an old revision of this page, as edited by Beetstra (talk | contribs) at 20:29, 10 November 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEMBL').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 20:29, 10 November 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEMBL').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)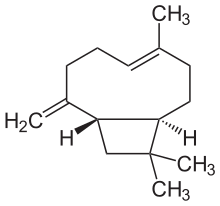
| |
| Names | |
|---|---|
| IUPAC names
4,11,11-trimethyl-8-methylene- bicycloundec-4-ene | |
| Other names
β-Caryophyllene; trans-(1R,9S)-8-Methylene-4,11,11-trimethylbicycloundec-4-ene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.588 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H24 |
| Molar mass | 204.36 g/mol |
| Density | 0.9052 g/cm |
| Boiling point | 262-264 °C; 129-130 °C (14 mm Hg) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Caryophyllene (Template:Pron-en), or (−)-β-caryophyllene, is a natural bicyclic sesquiterpene that is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of Syzygium aromaticum (cloves), the essential oil of hemp Cannabis sativa, rosemary Rosmarinus oficinalis, and hops. It is usually found as a mixture with isocaryophyllene (the cis double bond isomer) and α-humulene (obsolete name: α-caryophyllene), a ring-opened isomer. Caryophyllene is notable for having a cyclobutane ring, a rarity in nature.
Caryophyllene is one of the chemical compounds that contributes to the spiciness of black pepper. In a study conducted by Jürg Gertsch et al. from the Swiss Federal Institute of Technology (ETH Zurich), beta-caryophyllene was shown to selectively bind to the cannabinoid receptor type-2 (CB2) and to exert significant cannabimimetic antiinflammatory effects in mice. Since the widespread plant natural product beta-caryophyllene is an FDA approved food additive and ingested daily with food it is the first dietary cannabinoid. Whether this compound is able to modulate inflammatory processes in humans via the endocannabinoid system is yet unknown. Beta-caryophyllene does not bind to the centrally expressed cannabinoid receptor type-1 (CB1) and therefore does not exert psychomimetic effects.
The first total synthesis of caryophyllene in 1964 by E.J. Corey was considered one of the classic demonstrations of the possibilities of synthetic organic chemistry at the time.
Natural sources
The approximate quantity of caryophyllene in the essential oil of each source is given in square brackets ():
- Cannabis, hemp, marijuana (Cannabis sativa)
- Black Caraway (Carum nigrum)
- Cloves (Syzygium aromaticum)
- Hops (Humulus lupulus)
- Basil (Ocimum spp.)
- Oregano (Origanum vulgare)
- Black pepper (Piper nigrum)
- West African Pepper (Piper guineense)
- Rosemary (Rosmarinus officinalis)
- True cinnamon (Cinnamomum zeylanicum)
- Malabathrum (Cinnamomum tamala)
Compendial status
- Food Chemical Codex
Notes and references
- ^ Ghelardini C, Galeotti N, Di Cesare Mannelli L, Mazzanti G, Bartolini A (2001). "Local anaesthetic activity of beta-caryophyllene". Farmaco. 56 (5–7): 387–9. doi:10.1016/S0014-827X(01)01092-8. PMID 11482764.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gertsch J, Leonti M, Raduner S; et al. (2008). "Beta-caryophyllene is a dietary cannabinoid". Proceedings of the National Academy of Sciences of the United States of America. 105 (26): 9099–104. doi:10.1073/pnas.0803601105. PMC 2449371. PMID 18574142.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ormeño E, Baldy V, Ballini C, Fernandez C (2008). "Production and diversity of volatile terpenes from plants on calcareous and siliceous soils: effect of soil nutrients". J. Chem. Ecol. 34 (9): 1219–29. doi:10.1007/s10886-008-9515-2. PMID 18670820.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Glenn Tinseth, "Hop Aroma and Flavor", January/February 1993, Brewing Techniques. <http://realbeer.com/hops/aroma.html> Accessed July 21, 2010.
- Corey EJ, Mitra RB, Uda H (1964). "Total Synthesis of d,l-Caryophyllene and d,l-Isocaryophyllene". Journal of the American Chemical Society. 86 (3): 485–492. doi:10.1021/ja01057a040.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Mediavilla, Vito. "Essential oil of Cannabis sativa L. strains". International Hemp Association. Retrieved 11 July 2008.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Singh G, Marimuthu P, de Heluani CS, Catalan CA (2006). "Antioxidant and biocidal activities of Carum nigrum (seed) essential oil, oleoresin, and their selected components". J. Agric. Food Chem. 54 (1): 174–81. doi:10.1021/jf0518610. PMID 16390196.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Alma, M. Hakkı; Ertaş, Murat; Nitz, Siegfrie; Kollmannsberger, Hubert (2007). Lucia, Lucian A.; Hubbe, Martin A. (eds.). "Chemical composition and content of essential oil from the bud of cultivated Turkish clove" (PDF). BioResources. 2 (2). Raleigh, North Carolina, USA: North Carolina State University: 265–269. ISSN 1930-2126. Retrieved September 6, 2010.
The results showed that the essential oils mainly contained about 3.56% β-Caryophyllene
{{cite journal}}: Unknown parameter|month=ignored (help) - Wang G, Tian L, Aziz N; et al. (2008). "Terpene Biosynthesis in Glandular Trichomes of Hop". Plant Physiol. 148 (3): 1254–66. doi:10.1104/pp.108.125187. PMC 2577278. PMID 18775972.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Bernotienë, Genovaitë; Nivinskienë, Ona; Butkienë, Rita; Mockutë, Danutë (2004). "Chemical composition of essential oils of hops (Humulus lupulus L.) growing wild in Auktaitija" (PDF). Chemija. 2. 4. Vilnius, Lithuania: Lithuanian Academy of Sciences: 31–36. ISSN 0235-7216. Retrieved September 6, 2010.
{{cite journal}}: C1 control character in|title=at position 88 (help) - Zheljazkov VD, Cantrell CL, Tekwani B, Khan SI (2008). "Content, composition, and bioactivity of the essential oils of three basil genotypes as a function of harvesting". J. Agric. Food Chem. 56 (2): 380–5. doi:10.1021/jf0725629. PMID 18095647.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Silva, Maria Goretti de Vasconcelos; Matos, Francisco José de Abreu; Lopes, Paulo Roberto Oliveira; Silva, Fábio Oliveira; Holanda, Márcio Tavares (August 2, 2004). Cragg, Gordon M.; Bolzani, Vanderlan S.; Rao, G. S. R. Subba (eds.). "Composition of essential oils from three Ocimum species obtained by steam and microwave distillation and supercritical CO2 extraction" (PDF). Arkivoc. 2004 (vi). ARKAT USA, Inc.: 66–71. ISSN 1424-6376. Retrieved September 6, 2010.
- Harvala C, Menounos P, Argyriadou N (1987). "Essential Oil from Origanum dictamnus". Planta Med. 53 (1): 107–9. doi:10.1055/s-2006-962640. PMID 17268981.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Calvo-Irabien, L. M.; Yam-Puc, J. A.; Dzib, G.; Escalante-Erosa, F.; Peña-Rodriguez, L. M. (2009). "Effect of Postharvest Drying on the Composition of Mexican Oregano (Lippia graveolens) Essential Oil". Journal of Herbs, Spices & Medicinal Plants. 15 (3). London, UK: Taylor & Francis: 281–287. doi:10.1080/10496470903379001. ISSN 1540-3580.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Jirovetz L, Buchbauer G, Ngassoum MB, Geissler M (2002). "Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography-mass spectrometry and olfactometry". J Chromatogr A. 976 (1–2): 265–75. doi:10.1016/S0021-9673(02)00376-X. PMID 12462618.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Jamshidi, R.; Afzali, Z.; Afzali, D. (2009). "Chemical Composition of Hydrodistillation Essential Oil of Rosemary in Different Origins in Iran and Comparison with Other Countries" (PDF). American-Eurasian Journal of Agricultural & Environmental Sciences. 5 (1). Pakistan: IDOSI Publications: 78–81. ISSN 1990-4053. Retrieved September 6, 2010.
{{cite journal}}: Unknown parameter|month=ignored (help) - Kaul PN, Bhattacharya AK, Rao BRR; et al. (2003). "Volatile constituents of essential oils isolated from different parts of cinnamon (Cinnamomum zeylanicum Blume)". Journal of the Science of Food and Agriculture. 83 (1): 53–55. doi:10.1002/jsfa.1277.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Ahmed A, Choudhary MI, Farooq A; et al. (2000). "Essential oil constituents of the spice Cinnamomum tamala (Ham.) Nees & Eberm". Flavour and Fragrance Journal. 15 (6): 388–390. doi:10.1002/1099-1026(200011/12)15:6<388::AID-FFJ928>3.0.CO;2-F.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - The United States Pharmacopeial Convention. "Revisions to FCC, First Supplement". Retrieved 29 June 2009.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - Therapeutic Goods Administration. "Chemical substances" (PDF). Retrieved 29 June 2009.
External links
- "Some Proof that Marijuana is a Powerful Medicine", Aaron Rowe, Wired Magazine, June 29, 2008