This is an old revision of this page, as edited by The chemistds (talk | contribs) at 14:38, 18 November 2011 (added CSID, (Std)InChI & (Std)InChIKey). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 14:38, 18 November 2011 by The chemistds (talk | contribs) (added CSID, (Std)InChI & (Std)InChIKey)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)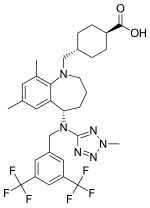
| |
| Names | |
|---|---|
| IUPAC name Trans-4-({(5S)-5-methyl}(2-methyl-2H-tetrazol-5- yl)amino]-7,9-dimethyl-2,3,4,5-tetrahydro-1H-benzazepin-1-yl}methyl) cyclohexanecarboxylic acid | |
| Other names LY2484595 | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.227.032 |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C31H36F6N6O2 |
| Molar mass | 638.659 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Evacetrapib is a drug under development by Eli Lilly & Company (investigational name LY2484595) that inhibits cholesterylester transfer protein, which transfers and thereby increases high-density lipoprotein and lowers low-density lipoprotein. It is thought that modifying lipoprotein levels modifies the risk of cardiovascular disease. The first CEPT inhibitor, torcetrapib, was unsuccesful because it increased levels of the hormone aldosterone and increased blood pressure, which led to excess cardiac events when it was studied. Evacetrapib does not have the same effect. When studied in a small clinical trial in people with elevated LDL and low HDL, significant improvements were noted in their lipid profile.
Together with anacetrapib and dalcetrapib, evacetrapib is the third CETP inhibitor currently being investigated. Despite the problems with torcetrapib, it is thought that CETP inhibitors are still likely to be useful in the treatment of dyslipidemia and the prevention of cardiovascular disease.
References
- ^ Cao G, Beyer TP, Zhang Y; et al. (2011). "Evacetrapib is a novel, potent, and selective inhibitor of cholesteryl ester transfer protein that elevates HDL cholesterol without inducing aldosterone or increasing blood pressure". J. Lipid Res. 52 (12): 2169–76. doi:10.1194/jlr.M018069. PMID 21957197.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Joy T, Hegele RA (2009). "The end of the road for CETP inhibitors after torcetrapib?". Curr. Opin. Cardiol. 24 (4): 364–71. doi:10.1097/HCO.0b013e32832ac166. PMID 19522058.
{{cite journal}}: Unknown parameter|month=ignored (help) - Nicholls SJ, Brewer HB, Kastelein JJ, Krueger KA, Wang MD, Shao M, Hu B, McErlean E, Nissen SE (2011). "Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol". JAMA. 306 (19): 2099–109. doi:10.1001/jama.2011.1649.
{{cite journal}}: CS1 maint: multiple names: authors list (link)