This is an old revision of this page, as edited by 70.137.157.59 (talk) at 00:14, 6 December 2011 (cite pmid). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 00:14, 6 December 2011 by 70.137.157.59 (talk) (cite pmid)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound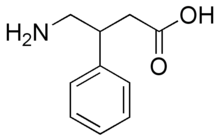 | |
| Clinical data | |
|---|---|
| Other names | Fenibut, Phenybut, PhGABA |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 5 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.800 |
| Chemical and physical data | |
| Formula | C10H13NO2 |
| Molar mass | 179.216 g/mol g·mol |
| 3D model (JSmol) | |
| Melting point | 253 °C (487 °F) |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
β-Phenyl-γ-aminobutyric acid, better known as phenibut or less commonly fenibut or phenybut, is a derivative of the naturally occurring inhibitory neurotransmitter γ-aminobutyric acid (GABA). The addition of a phenyl ring allows phenibut to cross the blood brain barrier. Phenibut is sold as a dietary supplement in the United States, while in Russia it is sold as a neuropsychotropic drug. It has been reported by some to possess nootropic actions for its ability to improve neurological functions, however other researchers had not previously observed these effects. However, it is generally accepted that phenibut has anxiolytic effects in both animal models and in humans. Phenibut was discovered in the Soviet Union in the 1960s, and has since been used there to treat a wide range of ailments including post-traumatic stress disorder, anxiety, and insomnia.
The name phenibut, along with many of the other names for the compound, comes directly from the chemical name for the compound, β-phenyl-γ-aminobutyric acid.
History
Phenibut was synthesized at the I. M. Herzen Leningrad Pedagogical Institute USSR and the Institute of Experimental Medicine, Academy of Medical Sciences USSR by Professor V. V. Perekalin's team.
Phenibut is mandated standard equipment in a Russian cosmonaut's medical kit. The use of "conventional" tranquilizers for stress and anxiety makes patients drowsy, which was deemed unacceptable for cosmonauts; phenibut, however, lowers stress levels without adversely affecting performance. In 1975 phenibut was included in the cosmonauts' kit for those who participated in the Apollo-Soyuz joint mission.
General information
In chemical structure, phenibut is a phenyl derivative of GABA. Although the calming action is similar to other GABA agonists, 4-amino-3-phenylbutyric acid binds the GABAB metabotropic receptor, the same site responsible for the sedative effects of baclofen (the para-chloro derivative of phenibut) and γ-hydroxybutyrate (GHB), although GHB also acts at the GHB receptor. There is dispute in the literature about whether or not Phenibut binds to the GABAA ionotropic receptor, which is responsible for the actions of the benzodiazepines, barbiturates, z-drugs, and for some of the effects of ethanol. According to Allikmetz and Ryage (1983) and Shulgina (1986) phenibut does bind to the GABAA receptor, but according to Lapin (2001) it does not. In the case of the former, it is argued that the GABAA binding only occurs at higher concentrations.
The literature that supports the nootropic effects of phenibut also suggest it elicits tranquilizing effects, reduction of stress and anxiety, improvement of impaired sleep, and the potentiation of the effects of tranquilizers, narcotics, and neuroleptics. It is also suggested to have an anticonvulsant effect, though studies on other GABAB agonists, such as the phenibut analogues baclofen and GHB, have shown them to act as potential convulsants. It should be noted, however, that GHB and baclofen (to a lesser extent) act on the convulsion-inducing GHB receptor, which phenibut does not.
Physical properties
Phenibut is a white crystalline powder and the taste is sour. It is very easily soluble in water, soluble in alcohol, and the pH of a 2.5% water solution is between about 2.3 and 2.7.
Doses
Commonly recommended doses are 250–1500 mg, twice daily, or as needed. This is up to the discretion of the health care practitioner as it pertains to patient needs. At higher doses above 40 mg/kg, a lowering of body temperature may occur along with muscle relaxation.
Chemistry
Structurally, phenibut is γ-aminobutyric acid with a phenyl group in the β position. Only the R enantiomer is biologically active. It has structural similarities to baclofen (lacking only a chlorine atom in the para-position of the phenyl group) and phenylethylamine.
Pharmacology
The pharmacological effects of phenibut are similar to baclofen, but less potent per milligram of dosage.
Phenibut exerts it effects by being an agonist at the metabotropic GABAB receptor, and at higher doses also at the ionotropic GABAA receptor.
Some studies found that 4-amino-3-phenylbutyric acid antagonizes the effects of phenylethylamine (PEA), while others found no effect on PEA induced anxiety.
Furthermore, phenibut has been shown to enhance levels of dopamine.
Contraindications and side effects
The literature reports that phenibut has almost no negative side effects, with only an increase in sleepiness observed, however this effect is not nearly as pronounced as with benzodiazepine usage. Tolerance has been reported with extended use of high doses (e.g. 5–10 grams) of 4-amino-3-phenylbutyric acid.
There are numerous reports of withdrawal symptoms on Internet blogs and a medically documented case of withdrawal involving severe anxiety, tremors agitation, psychosis, hallucinations and a complaint of insomnia similar to baclofen withdrawal, consistent with its GABAB agonist properties. There has been no systematic study of this problem.
Phenibut is not to be mixed with alcohol, sedatives, or prescription medication without consulting with a health-care professional.
Persons on MAO inhibitors or epilepsy medications like carbamazepine or oxcarbazepine should consult with their physician or pharmacist prior to supplementation with phenibut. Some evidence suggests that phenibut can modulate the function of some epilepsy medications.
References
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 11830761 , please use {{cite journal}} with
|pmid= 11830761instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 2431377 , please use {{cite journal}} with
|pmid= 2431377instead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 6705902 , please use {{cite journal}} with
|pmid= 6705902instead. - Slava Lapin (30 July 2009). From the Inside. Luniver Press. p. 209. ISBN 9781905986118. Retrieved 6 November 2010.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18275958 , please use {{cite journal}} with
|pmid= 18275958instead. -
Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7791129 , please use {{cite journal}} with
|pmid= 7791129instead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9235216 , please use {{cite journal}} with
|pmid= 9235216instead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 14162373 , please use {{cite journal}} with
|pmid= 14162373instead. - Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 4043364 , please use {{cite journal}} with
|pmid= 4043364instead. -
Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 1804703 , please use {{cite journal}} with
|pmid= 1804703instead. - Nelson, LS (2008). "Phenibut Withdrawal - A Novel 'Nutritional Supplement'". Clinical Toxicology. 46 (7): 605.
External links
Categories: