This is an old revision of this page, as edited by Jbstiller13 (talk | contribs) at 15:28, 5 February 2012. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 15:28, 5 February 2012 by Jbstiller13 (talk | contribs)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) | |
 | |
| Names | |
|---|---|
| IUPAC name 2,3-dihydroxybutanedioic acid | |
| Other names
2,3-dihydroxysuccinic acid threaric acid racemic acid uvic acid paratartaric acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.121.903 |
| E number | E334 (antioxidants, ...) |
| KEGG | |
| MeSH | tartaric+acid |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H6O6 (Basic formula) HO2CCH(OH)CH(OH)CO2H (Structural formula) |
| Molar mass | 150.087 g/mol |
| Appearance | white powder |
| Density | 1.79 g/mL (H2O) |
| Melting point | 171–174 °C (L or D-tartaric; pure) 206 °C (DL, racemic) 165-166°C ("meso-anhyrdous") 146–148 °C (meso-hydrous) |
| Solubility in water | 133 g/100ml (20 °C) |
| Acidity (pKa) | L(+) 25 °C : pKa1= 2.95 pKa2= 4.25 meso 25 °C: pKa1= 3.22 pKa2= 4.85 |
| Related compounds | |
| Other cations | Monosodium tartrate Disodium tartrate Monopotassium tartrate Dipotassium tartrate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tartaric acid is a white crystalline diprotic organic acid. It occurs naturally in many plants, particularly grapes, bananas, and tamarinds; is commonly combined with baking soda to function as a leavening agent in recipes, and is one of the main acids found in wine. It is added to other foods to give a sour taste, and is used as an antioxidant. Salts of tartaric acid are known as tartrates. It is a dihydroxyl derivative of succinic acid.
Tartaric acid was first isolated from potassium tartrate, known to the ancients as tartar, c. 800 by the alchemist Jabir ibn Hayyan. The modern process was developed in 1769 by the Swedish chemist Carl Wilhelm Scheele.
Tartaric acid played an important role in the discovery of chemical chirality. This property of tartaric acid was first observed in 1832 by Jean Baptiste Biot, who observed its ability to rotate polarized light. Louis Pasteur continued this research in 1847 by investigating the shapes of ammonium sodium tartrate crystals, which he found to be chiral. By manually sorting the differently shaped crystals under magnification, Pasteur was the first to produce a pure sample of levotartaric acid.
Stereochemistry
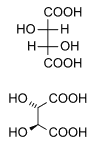
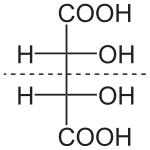
Naturally occurring tartaric acid is chiral, meaning that it has molecules that are non-superimposable on their mirror-images. It is a useful raw material in organic chemistry for the synthesis of other chiral molecules. The naturally occurring form of the acid is L-(+)-tartaric acid or dextrotartaric acid. The mirror-image (enantiomeric) form, levotartaric acid or D-(−)-tartaric acid, and the achiral form, mesotartaric acid, can be made artificially. Note, that the dextro and levo prefixes are not related to the D/L configuration (which is derived rather indirectly from their structural relation to D- or L-glyceraldehyde), but to the orientation of the optical rotation, (+) = dextrorotatory, (−) = levorotatory. Sometimes, instead of capital letters, small italic d and l are used. They are abbreviations of dextro- and levo- and, nowadays, should not be used. Levotartaric and dextrotartaric acid are enantiomers, mesotartaric acid is a diastereomer of both of them.
A rarely occurring optically inactive form of tartaric acid, DL-tartaric acid is a 1:1 mixture of the levo and dextro forms. It is distinct from mesotartaric acid and was called racemic acid (from Latin racemus – "a bunch of grapes"). The word racemic later changed its meaning, becoming a general term for 1:1 enantiomeric mixtures – racemates.
Tartaric acid is used to prevent copper(II) ions from reacting with the hydroxide ions present in the preparation of copper(I) oxide. Copper(I) oxide is a reddish brown solid, and is produced by the reduction of a Cu(II) salt with an aldehyde, in an alkaline solution.
| levotartaric acid (D-(−)-tartaric acid) |
dextrotartaric acid (L-(+)-tartaric acid) |
mesotartaric acid |
|---|---|---|

|
| |
|
DL-tartaric acid (racemic acid) | ||
| Forms of tartaric acid | ||||||
|---|---|---|---|---|---|---|
| Common name | tartaric acid | levotartaric acid | dextrotartaric acid | mesotartaric acid | racemic acid | |
| Synonyms | D-(S,S)-(−)-tartaric acid unnatural isomer |
L-(R,R)-(+)-tartaric acid natural isomer |
(2R,3S)-tartaric acid | DL-(S,S/R,R)-(±)-tartaric acid | ||
| PubChem | CID 875 from PubChem | CID 439655 from PubChem | CID 444305 from PubChem | CID 78956 from PubChem | CID 5851 from PubChem | |
| EINECS number | 205-695-6 | 201-766-0 | 205-696-1 | 205-105-7 | ||
| CAS number | 526-83-0 | 147-71-7 | 87-69-4 | 147-73-9 | 133-37-9 | |
Derivatives


Important derivatives of tartaric acid include its salts, cream of tartar (potassium bitartrate), Rochelle salt (potassium sodium tartrate, a mild laxative), and tartar emetic (antimony potassium tartrate). Diisopropyl tartrate is used as a catalyst in asymmetric synthesis.
Tartaric acid is a muscle toxin, which works by inhibiting the production of malic acid, and in high doses causes paralysis and death. The median lethal dose (LD50) is about 7.5 grams/kg for a human, ~5.3 grams/kg for rabbits and ~4.4 grams/kg for mice. Given this figure, it would take over 500 grams (18 oz) to kill a person weighing 70 kilograms (150 lb), and so it may be safely included in many foods, especially sour-tasting sweets. As a food additive, tartaric acid is used as an antioxidant with E number E334, tartrates are other additives serving as antioxidants or emulsifiers.
When cream of tartar is added to water, a suspension results which serves to clean copper coins very well, as the tartrate solution can dissolve the layer of copper(II) oxide present on the surface of the coin. The resulting copper(II)-tartrate complex is easily soluble in water.
Tartaric acid in wine
See also: Acids in wine
Tartaric acid may be most immediately recognizable to wine drinkers as the source of "wine diamonds", the small potassium bitartrate crystals that sometimes form spontaneously on the cork. These "tartrates" are harmless, despite sometimes being mistaken for broken glass, and are prevented in many wines through cold stabilization. The tartrates that remain on the inside of aging barrels were at one time a major industrial source of potassium bitartrate.
However, tartaric acid plays an important role chemically, lowering the pH of fermenting "must" to a level where many undesirable spoilage bacteria cannot live, and acting as a preservative after fermentation. In the mouth, tartaric acid provides some of the tartness in the wine, although citric and malic acids also play a role.
References
- Tartaric Acid – Compound Summary, PubChem.
- Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton, Florida: CRC Press. ISBN 0-8493-0486-5.
- Lisa Solieri, Paolo Giudici (2009). Vinegars of the World. Springer. p. 29. ISBN 8847008654.
- L. Pasteur (1848) "Mémoire sur la relation qui peut exister entre la forme cristalline et la composition chimique, et sur la cause de la polarisation rotatoire" (Memoir on the relationship which can exist between crystalline form and chemical composition, and on the cause of rotary polarization)," Comptes rendus de l'Académie des sciences (Paris), vol. 26, pp. 535–538.
- L. Pasteur (1848) "Sur les relations qui peuvent exister entre la forme cristalline, la composition chimique et le sens de la polarisation rotatoire" (On the relations that can exist between crystalline form, and chemical composition, and the sense of rotary polarization), Annales de Chimie et de Physique, 3rd series, vol. 24, no. 6, pages 442–459.
- George B. Kauffman and Robin D. Myers (1998). "Pasteur's resolution of racemic acid: A sesquicentennial retrospect and a new translation" (PDF). The Chemical Educator. 3 (6): 1–4. doi:10.1007/s00897980257a.
- H. D. Flack (2009). "Louis Pasteur's discovery of molecular chirality and spontaneous resolution in 1848, together with a complete review of his crystallographic and chemical work" (PDF). Acta Crystallographica A. 65 (5): 371–389. doi:10.1107/S0108767309024088. PMID 19687573.
- J. M. McBride's Yale lecture on history of stereochemistry of tartaric acid, the D/L and R/S systems
- various (2007-07-23). Organic Chemistry. Global Media. p. 65. ISBN 9788189940768. Retrieved 2010-06-05.
- "(WO/2008/022994) Use of azabicyclo hexane derivatives".
- Zalkin, Allan; Templeton, David H.; Ueki, Tatzuo (1973). "Crystal structure of l-tris(1,10-phenathroline)iron(II) bis(antimony(III) d-tartrate) octahydrate". Inorganic Chemistry. 12 (7): 1641. doi:10.1021/ic50125a033.
- Haq, I; Khan, C (1982). "Hazards of a traditional eye-cosmetic--SURMA". JPMA. the Journal of the Pakistan Medical Association. 32 (1): 7–8. PMID 6804665.
- McCallum, RI (1977). "President's address. Observations upon antimony". Proceedings of the Royal Society of Medicine. 70 (11): 756–63. PMC 1543508. PMID 341167.
- Alfred Swaine Taylor, Edward Hartshorne (1861). Medical jurisprudence. Blanchard and Lea. p. 61.
{{cite book}}: Unknown parameter|unused_data=ignored (help) - Joseph A. Maga, Anthony T. Tu (1995). Food additive toxicology. CRC Press. pp. 137–138. ISBN 0824792459.