This is an old revision of this page, as edited by Beetstra (talk | contribs) at 12:54, 15 February 2012 (Saving copy of the {{chembox}} taken from revid 472883278 of page Barium_sulfate for the Chem/Drugbox validation project (updated: '').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 12:54, 15 February 2012 by Beetstra (talk | contribs) (Saving copy of the {{chembox}} taken from revid 472883278 of page Barium_sulfate for the Chem/Drugbox validation project (updated: '').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This page contains a copy of the infobox ({{chembox}}) taken from revid 472883278 of page Barium_sulfate with values updated to verified values. |

| |||
| |||
| Identifiers | |||
|---|---|---|---|
| CAS Number | |||
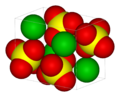
| 3D model (JSmol) | |||
| ChemSpider | |||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | BaSO4 | ||
| Molar mass | 233.43 g/mol | ||
| Appearance | white crystalline | ||
| Odor | odorless | ||
| Density | 4.5 g/cm | ||
| Melting point | 1345 °C | ||
| Boiling point | 1600 °C (decomp) | ||
| Solubility in water | 0.0002448 g/100 mL (20 °C) 0.000285 g/100 mL (30 °C) | ||
| Solubility product (Ksp) | 1.0842 × 10 (25 °C) | ||
| Solubility | insoluble in alcohol, soluble in concentrated sulfuric acid | ||
| Refractive index (nD) | 1.64 | ||
| Thermochemistry | |||
| Std molar entropy (S298) |
132 J·mol·K | ||
| Std enthalpy of formation (ΔfH298) |
−1465 kJ·mol | ||
| Pharmacology | |||
| Pharmacokinetics: | |||
| Bioavailability | negligible orally | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound
- CRC Handbook of Chemistry and Physics. CRC Press. 2004,85th Edition. pp. 4–45. ISBN 0-8493-0485-7.
{{cite book}}: Check date values in:|date=(help) - Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. ISBN 061894690X.
- Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. ISBN 061894690X.