This is an old revision of this page, as edited by Beetstra (talk | contribs) at 04:40, 17 February 2012 (Saving copy of the {{chembox}} taken from revid 477287673 of page Sulfur_hexafluoride for the Chem/Drugbox validation project (updated: 'UNII', 'KEGG').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 04:40, 17 February 2012 by Beetstra (talk | contribs) (Saving copy of the {{chembox}} taken from revid 477287673 of page Sulfur_hexafluoride for the Chem/Drugbox validation project (updated: 'UNII', 'KEGG').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This page contains a copy of the infobox ({{chembox}}) taken from revid 477287673 of page Sulfur_hexafluoride with values updated to verified values. |
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name Sulfur hexafluoride | |||
| Systematic IUPAC name Hexafluoro-λ-sulfane | |||
| Other names
Elagas Esaflon | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
| Gmelin Reference | 2752 | ||
| MeSH | Sulfur+hexafluoride | ||
| PubChem CID | |||
| RTECS number |
| ||
| UN number | 1080 | ||
InChI
| |||
SMILES
| |||
| Properties | |||
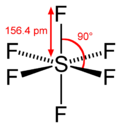
| Chemical formula | F6S | ||
| Molar mass | 146.05 g·mol | ||
| Appearance | Colorless, odorless gas | ||
| Density | 6.17 g/l | ||
| Boiling point | −64 °C; −83 °F; 209 K | ||
| Vapor pressure | 2.9 kPa (at 21.1°C) | ||
| Structure | |||
| Crystal structure | Orthorhombic, oP28 | ||
| Space group | Oh | ||
| Coordination geometry | Orthogonal hexagonal | ||
| Molecular shape | Octahedral | ||
| Dipole moment | 0 D | ||
| Thermochemistry | |||
| Std molar entropy (S298) |
292 J·mol·K | ||
| Std enthalpy of formation (ΔfH298) |
−1209 kJ·mol | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound
- "Sulfur Hexafluoride - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ^ Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 061894690X.