This is an old revision of this page, as edited by Beetstra (talk | contribs) at 11:02, 20 February 2012 (Saving copy of the {{drugbox}} taken from revid 469541507 of page Treprostinil for the Chem/Drugbox validation project (updated: 'ChEMBL', 'CAS_number').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 11:02, 20 February 2012 by Beetstra (talk | contribs) (Saving copy of the {{drugbox}} taken from revid 469541507 of page Treprostinil for the Chem/Drugbox validation project (updated: 'ChEMBL', 'CAS_number').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)| This page contains a copy of the infobox ({{drugbox}}) taken from revid 469541507 of page Treprostinil with values updated to verified values. |
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Remodulin, Tyvaso |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a600042 |
| Pregnancy category |
|
| Routes of administration | Subcutaneous or intravenous infusion, inhalation |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | approximately 100% |
| Metabolism | Treprostinil is substantially metabolized by the liver, but the involved enzymes are not currently known. Five metabolites (HU1 through HU5) have been described thus far. Based on the results of in vitro human hepatic cytochrome P450 studies, Remodulin does not inhibit CYP-1A2, 2C9, 2C19, 2D6, 2E1, or 3A. Whether Remodulin induces these enzymes has not been studied. |
| Elimination half-life | 4 hours |
| Excretion | Urine (79 % of administered dose is excreted in the urine as 4% unchanged drug and 64% as identified metabolites); feces (13%) |
| Identifiers | |
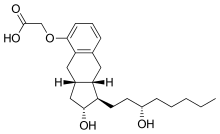
IUPAC name
| |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C23H34O5 |
| Molar mass | relative molecular weight is 390.52 g/mol. g·mol |
InChI
| |
| (what is this?) (verify) | |