This is an old revision of this page, as edited by Leyo (talk | contribs) at 08:13, 31 May 2013 (corr, see talk page). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 08:13, 31 May 2013 by Leyo (talk | contribs) (corr, see talk page)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)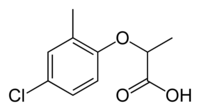 | |
| Names | |
|---|---|
| IUPAC name (RS)-2-(4-Chloro-2-methylphenoxy)propanoic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.002.060 |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C10H11ClO3 |
| Molar mass | 214.65 g·mol |
| Appearance | Solid |
| Melting point | 94–95 °C |
| Boiling point | decomposes |
| Solubility in water | 900 mg/L(20 °C) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Xn, N |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Mecoprop, or methylchlorophenoxypropionic acid (MCPP), is a common general use herbicide found in many household weed killers and "weed-and-feed" type lawn fertilizers. It is primarily used to control broadleaf weeds. It is often used in combination with other chemically related herbicides such as 2,4-D, dicamba, and MCPA.
The United States Environmental Protection Agency has classified mecoprop as toxicity class III - slightly toxic.
Mecoprop is a mixture of two stereoisomers, with the (R)-(+)-enantiomer ("Mecoprop-P", "Duplosan KV") possessing the herbicidal activity.

See also
References
- Merck Index, 11th Edition, 5666.
- ^ Record of Mecoprop in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 8 September 2008.
- Template:HPD
- ^ Mecoprop at EXTOXNET
- G. Smith, C. H. L. Kennard, A. H. White and P. G. Hodgson (1980). "(±)-2-(4-Chloro-2-methylphenoxy)propionic acid (mecoprop)". Acta Cryst. B36 (4): 992–994. doi:10.1107/S0567740880005134.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
External links
- Mecoprop Pesticide Information Profile - Extension Toxicology Network
- Mecoprop at AlanWood.net
- Mecoprop at pesticideinfo.org
- Mecoprop at coastalwiki.org