This is an old revision of this page, as edited by 137.132.3.12 (talk) at 06:41, 30 October 2006. The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 06:41, 30 October 2006 by 137.132.3.12 (talk)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) For other uses, see China (disambiguation).
Color or colour (see spelling differences) is the visual perceptual property corresponding in humans to the categories called red, yellow, white, etc. Color derives from the spectrum of light (distribution of light energy versus wavelength) interacting in the eye with the spectral sensitivities of the light receptors. Color categories and physical specifications of color are also associated with objects, materials, light sources, etc., based on their physical properties such as light absorption, reflection, or emission spectra.
Typically, only features of the composition of light that are detectable by humans (wavelength spectrum from 400 nm to 700 nm, roughly) are included, thereby objectively relating the psychological phenomenon of color to its physical specification.
Since perception of color stems from the varying sensitivity of different types of cone cells in the retina to different parts of the spectrum, colors may be defined and quantified by the degree to which they stimulate these cells. These physical or physiological quantifications of color, however, do not fully explain the psychophysical perception of color appearance.
The science of color is sometimes called chromatics. It includes the perception of color by the human eye and brain, the origin of color in materials, color theory in art, and the physics of electromagnetic radiation in the visible range (that is, what we commonly refer to simply as light).
Physics of color
| color | wavelength interval | frequency interval |
|---|---|---|
| red | ~ 625–740 nm | ~ 480–405 THz |
| orange | ~ 590–625 nm | ~ 510–480 THz |
| yellow | ~ 565–590 nm | ~ 530–510 THz |
| green | ~ 500–565 nm | ~ 600–530 THz |
| cyan | ~ 485–500 nm | ~ 620–600 THz |
| blue | ~ 440–485 nm | ~ 680–620 THz |
| violet | ~ 380–440 nm | ~ 790–680 THz |
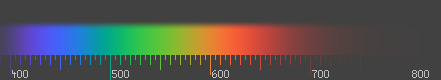

| Color | /nm | /10 Hz | /10 cm | /eV | /kJ mol |
|---|---|---|---|---|---|
| Infrared | >1000 | <3.00 | <1.00 | <1.24 | <120 |
| Red | 700 | 4.28 | 1.43 | 1.77 | 171 |
| Orange | 620 | 4.84 | 1.61 | 2.00 | 193 |
| Yellow | 580 | 5.17 | 1.72 | 2.14 | 206 |
| Green | 530 | 5.66 | 1.89 | 2.34 | 226 |
| Blue | 470 | 6.38 | 2.13 | 2.64 | 254 |
| Violet | 420 | 7.14 | 2.38 | 2.95 | 285 |
| Near ultraviolet | 300 | 10.0 | 3.33 | 4.15 | 400 |
| Far ultraviolet | <200 | >15.0 | >5.00 | >6.20 | >598 |
Electromagnetic radiation is characterized by its wavelength (or frequency) and its intensity. When the wavelength is within the visible spectrum (the range of wavelengths humans can perceive, approximately from 380 nm to 740 nm), it is known as "visible light."
A given light source may emit light at many different wavelengths (and most do); its spectrum is then a distribution giving its intensity at each wavelength. Although the spectrum of light arriving at the eye from a given direction determines the color perceived in that direction, there are many more possible spectral combinations than color sensations. In fact, one may formally define a color as a class of spectra that give rise to the same color sensation, although such classes would vary widely among different species, and to a lesser extent among individuals within the same species. In each such class the members are called metamers of the color in question.
Spectral colors
The familiar colors of the rainbow in the spectrum – named from the Latin word for appearance or apparition by Isaac Newton in 1671 – contains all those colors that can be produced by visible light of a single wavelength only, the pure spectral or monochromatic colors. The color table at right shows approximate frequencies (in terahertz) and wavelengths (in nanometers) for various pure spectral colors. The wavelengths are measured in vacuum (see refraction).
The color table should not be interpreted as a definitive list – the pure spectral colors form a continuous spectrum, and how it is divided into distinct colors is a matter of culture, taste, and language. Newton added a seventh color, indigo, between blue and violet, but most people are not able to distinguish it and most color scientists do not recognize it as a separate color; it is sometimes designated as wavelengths of 420–440 nm. Furthermore, the intensity of a spectral color may alter its perception considerably; for example, a low-intensity orange-yellow is brown, and a low-intensity yellow-green is olive-green.
As discussed in the section on color vision, a light source need not actually be of one single wavelength to be perceived as a pure spectral color.
For discussion of non-spectral colors, see below.
Color of objects
Surfaces appear to have the color of the light leaving them in the direction of the eye. Since the composition of this light may depend on the orientation of the surface and lighting conditions, the perceived color of an object also depends on these factors. However, some generalizations can be drawn.
Light arriving at an opaque surface is either reflected "specularly" (that is, in the manner of a mirror), scattered (that is, reflected with diffuse scattering), or absorbed – or some combination of these.
Opaque objects that do not reflect specularly (which tend to have rough surfaces) have their color determined by which wavelengths of light they scatter more and which they scatter less (with the light that is not scattered being absorbed). If objects scatter all wavelengths, they appear white. If they absorb all wavelengths, they appear black.
Opaque objects that specularly reflect light of different wavelengths with different efficiencies look like mirrors tinted with colors determined by those differences. An object that reflects some fraction of impinging light and absorbs the rest may look black but also be faintly reflective; examples are black objects coated with layers of enamel or lacquer.
Objects that transmit light are either translucent (scattering the transmitted light) or transparent (not scattering the transmitted light). If they also absorb (or reflect) light of varying wavelengths differentially, they appear tinted with a color determined by the nature of that absorption (or that reflectance).
Objects may emit light that they generate themselves, rather than merely reflecting or transmitting light. They may do so because of their elevated temperature (they are then said to be incandescent), as a result of certain chemical reactions (a phenomenon called chemoluminescence), or for other reasons (see the articles Phosphorescence and List of light sources).
Objects may absorb light and then as a consequence emit light that has different properties. They are then called fluorescent (if light is emitted only while light is absorbed) or phosphorescent (if light is emitted even after light ceases to be absorbed; this term is also sometimes loosely applied to light emitted due to chemical reactions).
For further treatment of the color of objects, see the section Structural color, below.
To summarize, the color of an object is a complex result of its surface properties, its transmission properties, and its emission properties, all of which factors contribute to the mix of wavelengths in the light leaving the surface of the object. The perceived color is then further conditioned by the nature of the ambient illumination, and by the color properties of other objects nearby (see the article Color constancy); and finally, by the permanent and transient characteristics of the perceiving eye and brain.
Color perception

Color in the eye
Main article: Color visionThe ability of the human eye to distinguish colors is based upon the varying sensitivity of different cells in the retina to light of different wavelengths. The retina contains three types of color receptor cells, or cones. One type, relatively distinct from the other two, is most responsive to light that we perceive as violet, with wavelengths around 420 nm. (Cones of this type are sometimes called short-wavelength cones, S cones, or, misleadingly, blue cones.) The other two types are closely related genetically and chemically. One of them (sometimes called long-wavelength cones, L cones, or, misleadingly, red cones) is most sensitive to light we perceive as yellowish-green, with wavelengths around 564 nm; the other type (sometimes called middle-wavelength cones, M cones, or misleadingly, green cones) is most sensitive to light perceived as green, with wavelengths around 534 nm.
Light, no matter how complex its composition of wavelengths, is reduced to three color components by the eye. For each location in the visual field, the three types of cones yield three signals based on the extent to which each is stimulated. These values are sometimes called tristimulus values.
The response curve as a function of wavelength for each type of cone is illustrated above. Because the curves overlap, some tristimulus values do not occur for any incoming light combination. For example, it is not possible to stimulate only the mid-wavelength/"green" cones; the other cones will inevitably be stimulated to some degree at the same time. The set of all possible tristimulus values determines the human color space. It has been estimated that humans can distinguish roughly 10 million different colors.
The other type of light-sensitive cell in the eye, the rod, has a different response curve. In normal situations, when light is bright enough to strongly stimulate the cones, rods play virtually no role in vision at all. On the other hand, in dim light, the cones are understimulated leaving only the signal from the rods, resulting in a monochromatic response. (Furthermore, the rods are barely sensitive to light in the "red" range.) In certain conditions of intermediate illumination, the rod response and a weak cone response can together result in color discriminations not accounted for by cone responses alone.
Color in the brain
While the mechanisms of color vision at the level of the retina are well-described in terms of tristimulus values (see above), color processing after that point is organized differently. A dominant theory of color vision proposes that color information is transmitted out of the eye by three opponent processes, or opponent channels, each constructed from the raw output of the cones: a red-green channel, a blue-yellow channel and a black-white "luminance" channel. This theory has been supported by neurobiology, and accounts for the structure of our subjective color experience. Specifically, it explains why we cannot perceive a "reddish green" or "yellowish blue," and it predicts the color wheel: it is the collection of colors for which at least one of the two color channels measures a value at one of its extremes.
The exact nature of color perception beyond the processing already described, and indeed the status of color as a feature of the perceived world or rather as a feature of our perception of the world, is a matter of complex and continuing philosophical dispute (see qualia).
Development of theories of color vision
Although Aristotle and other ancient scientists speculated on the nature of light and color vision, it was not until Newton that light was correctly identified as the source of the color sensation. Goethe studied the theory of colors, and in 1801 Thomas Young proposed his trichromatic theory which was later refined by Hermann von Helmholtz. That theory was ultimately confirmed scientifically in the 1960s.
In 1931, an international group of experts known as the Commission Internationale d'Eclairage (CIE) developed a mathematical color model, which mapped out the space of observable colors and assigned a set of three numbers to each.
Nonstandard color perception
If one or more types of a person's color-sensing cones are missing or less responsive than normal to incoming light, that person can distinguish fewer colors and is said to be color deficient or color blind (though this latter term can be misleading; almost all color deficient individuals can distinguish at least some colors). Some kinds of color deficiency are caused by anomalies in the number or nature of cones in the retina. Others (like central or cortical achromatopsia) are caused by neural anomalies in those parts of the brain where visual processing takes place.
While most humans are trichromatic (having three types of color receptors), many animals, known as tetrachromats, have four types. These include some species of spiders, most marsupials, birds, reptiles, and many species of fish. Other species are sensitive to only two axes of color or do not perceive color at all; these are called dichromats and monochromats respectively. A distinction is made between retinal tetrachromacy (having four pigments in cone cells in the retina, compared to three in trichromats) and functional tetrachromacy (having the ability to make enhanced color discriminations based on that retinal difference). As many as a half of all women, but only a small percentage of men, are retinal tetrachromats. The phenomenon arises when an individual receives two slightly different copies of the gene for either the medium- or long-wavelength cones (which are carried on the x-chromosome). For some of these retinal tetrachromats, color discriminations are enhanced, making them functional tetrachromats.
In certain forms of synesthesia, perceiving letters and numbers (grapheme → color synesthesia) or hearing musical sounds (music → color synesthesia) will lead to the unusual additional experiences of seeing colors. Behavioral and functional neuroimaging experiments have demonstrated that these color experiences lead to changes behavioral tasks and lead to increased activation of brain regions involved in color perception, thus demonstrating their reality, and similarity to real color percepts, albeit evoked through a non-standard route.
Afterimages
After exposure to strong light in their sensitivity range, photoreceptors of a given type become desensitized. For a few seconds after the light ceases, they will continue to signal less strongly than they otherwise would. Colors observed during that period will appear to lack the color component detected by the desensitized photoreceptors. This effect is responsible for the phenomenon of afterimages, in which the eye may continue to see a bright figure after looking away from it, but in a complementary color.
Afterimage effects have also been utilized by artists, including Vincent van Gogh.
Color constancy
There is an interesting phenomenon which occurs when an artist uses a limited color palette: the eye tends to compensate by seeing any grey or neutral color as the color which is missing from the color wheel. E.g.: in a limited palette consisting of red, yellow, black and white, a mixture of yellow and black will appear as a variety of green, a mixture of red and black will appear as a variety of purple, and pure grey will appear bluish.
The trichromatric theory discussed above is strictly true only if the whole scene seen by the eye is of one and the same color, which of course is unrealistic. In reality, the brain compares the various colors in a scene, in order to eliminate the effects of the illumination. If a scene is illuminated with one light, and then with another, as long as the difference between the light sources stays within a reasonable range, the colors of the scene will nevertheless appear constant to us. This was studied by Edwin Land in the 1970s and led to his retinex theory of color constancy.
Color naming
Different cultures have different terms for colors, and may also assign some color names to slightly different parts of the spectrum: for instance, the han character 青 (rendered as qīng in Mandarin and ao in Japanese) has a meaning that covers both blue and green; blue and green are traditionally considered shades of "青." In more contemporary terms, they are 藍 (lán, in Mandarin) and 綠 (lǜ, in Mandarin) respectively. For example, in Japan, although the traffic lights have the same colored lights that other countries have, the green light is called using the same word for blue, "aoi," because green is considered a shade of blue.
Similarly, languages are selective when deciding which hues are split into different colors on the basis of how light or dark they are. Apart from the black-grey-white continuum, English splits some hues into several distinct colors according to lightness: such as red and pink or orange and brown. To English speakers, these pairs of colors, which are objectively no more different from one another than light green and dark green, are conceived as totally different. A Russian will make the same red-pink and orange-brown distinctions, but will also make a further distinction between sinij and goluboj, which English speakers would simply call dark and light blue. To Russian speakers, sinij and goluboj are as separate as red and pink or orange and brown.
Color terms evolve. It is argued that there are a limited number of universal "basic color terms" which begin to be used by individual cultures in a relatively fixed order. For example, a culture would start with only two terms, meaning roughly 'dark' (covering black, dark colors and cold colors such as blue ) and 'bright' (covering white, light colors and warm colors such as red), before adding more specific color names, in the order of red; green and/or yellow; blue; brown; and orange, pink, purple and/or gray. Older arguments for this theory also stipulated that the acquisition and use of basic color terms further along the evolutionary order indicated a more complex culture with more highly developed technology.
A somewhat dated example of a universal color categories theory is Basic Color Terms: Their Universality and Evolution (1969) by Brent Berlin and Paul Kay. A more recent example of a linguistic determinism theory might be Is color categorisation universal? New evidence from a stone-age culture (1999) by Jules Davidoff et al. The idea of linguistically determined color categories is often used as evidence for the Sapir-Whorf hypothesis (Language, Thought and Reality (1956) by Benjamin Lee Whorf).
Additionally, different colors are often associated with different emotional states, values or groups, but these associations can vary between cultures. In one system, red is considered to motivate action; orange and purple are related to spirituality; yellow cheers; green creates cosiness and warmth; blue relaxes; and white is associated with either purity or death. These associations are described more fully in the individual color pages, and under color psychology.
See also: National colors
Health effects
When the color spectrum of artificial lighting is mismatched to that of sunlight, material health effects may arise including increased incidence of headache. This phenomenon is often coupled with adverse effects of over-illumination, since many of the same interior spaces that have color mismatch also have higher light intensity than desirable for the task being conducted in that space.
Measurement and reproduction of color
Relation to spectral colors
Most light sources are mixtures of various wavelengths of light. However, many such sources can still have a spectral color insofar as the eye cannot distinguish them from monochromatic sources. For example, most computer displays reproduce the spectral color orange as a combination of red and green light; it appears orange because the red and green are mixed in the right proportions to allow the eye's red and green cones to respond the way they do to orange.
A useful concept in understanding the perceived color of a non-monochromatic light source is the dominant wavelength, which identifies the single wavelength of light which produces a sensation most similar to the light source. Dominant wavelength is roughly akin to hue.
Of course, there are many color perceptions that by definition cannot be pure spectral colors due to desaturation or because they are purples (mixtures of red and violet light, from opposite ends of the spectrum). Some examples of necessarily non-spectral colors are the achromatic colors (black, gray and white) and colors such as pink, tan, and magenta.

Two different light spectra which have the same effect on the three color receptors in the human eye will be perceived as the same color. This is exemplified by the white light that is emitted by fluorescent lamps, which typically has a spectrum consisting of a few narrow bands, while daylight has a continuous spectrum. The human eye cannot tell the difference between such light spectra just by looking into the light source, although reflected colors from objects can look different. (This is often exploited e.g. to make fruit or tomatoes look more brightly red in shops.)
Similarly, most human color perceptions can be generated by a mixture of three colors called primaries. This is used to reproduce color scenes in photography, printing, television and other media. There are a number of methods or color spaces for specifying a color in terms of three particular primary colors. Each method has its advantages and disadvantages depending on the particular application.
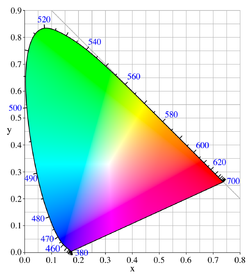
No mixture of colors, though, can produce a fully pure color perceived as completely identical to a spectral color, although one can get very close for the longer wavelengths, where the chromaticity diagram above has a nearly straight edge. For example, mixing green light (530 nm) and blue light (460 nm) produces cyan light that is slightly desaturated, because response of the red color receptor would be greater to the green and blue light in the mixture than it would be to a pure cyan light at 485 nm that has the same intensity as the mixture of blue and green.
Because of this, and because the primaries in color printing systems generally are not pure themselves, the colors reproduced are never perfectly saturated colors, and so spectral colors cannot be matched exactly. However, natural scenes rarely contain fully saturated colors, thus such scenes can usually be approximated well by these systems. The range of colors that can be reproduced with a given color reproduction system is called the gamut. The CIE chromaticity diagram can be used to describe the gamut.
Another problem with color reproduction systems is connected with the acquisition devices, like cameras or scanners. The characteristics of the color sensors in the devices are often very far from the characteristics of the receptors in the human eye. In effect, acquisition of colors that have some special, often very "jagged," spectra caused for example by unusual lighting of the photographed scene can be relatively poor.
Species that have color receptors different from humans, e. g. birds that may have four receptors, can differentiate some colors that look the same to a human. In such cases, a color reproduction system 'tuned' to a human with normal color vision may give very inaccurate results for the other observers.
The next problem is different color response of different devices. For color information stored and transferred in a digital form, color management technique based on color profiles attached to color data and to devices with different color response helps to avoid deformations of the reproduced colors. The technique works only for colors in gamut of the particular devices, e.g. it can still happen that your monitor is not able to show you real color of your goldfish even if your camera can receive and store the color information properly and vice versa.
Pigments and reflective media
Main article: PigmentPigments are chemicals that selectively absorb and reflect different spectra of light. When a surface is painted with a pigment, light hitting the surface is reflected, minus some wavelengths. This subtraction of wavelengths produces the appearance of different colors. Most paints are a blend of several chemical pigments, intended to produce a reflection of a given color.
Pigment manufacturers assume the source light will be white, or of roughly equal intensity across the spectrum. If the light isn't a pure white source (as in the case of nearly all forms of artificial lighting), the resulting spectrum will appear a slightly different color. Red paint, viewed under blue light, may appear black. Red paint is red because it reflects only the red components of the spectrum. Blue light, containing none of these, will create no reflection from red paint, creating the appearance of black.
Structural color
Structural colors are colors which are caused by interference effects rather than pigment. Colors are produced when a material is scored with fine parallel lines, formed of one or more thin parallel layers, or otherwise composed of microstructures on the scale of the color's wavelength. If the microstructures are spaced randomly, light of shorter wavelengths will be scattered preferentially to produce Tyndall effect colors: the blue of the sky, aerogel of opals, and the blue of human irises. If the microstructures are aligned in arrays, for example the array of pits in a CD, they behave as a diffraction grating, the grating reflects different wavelengths in different directions due to interference phenomena, separating white light into colors. If the structure is one or more thin layers then it will reflect some wavelengths and transmit others, depending on the thickness of the layer(s).
Structural color is responsible for the blues and greens of many bird feathers (example, blue jay feathers) as well as certain butterfly wings and beetle shells. Variations in the pattern's spacing often give rise to an iridescent effect, as seen in peacock feathers, soap bubbles, films of oil, and mother of pearl, because the reflected color depends upon the viewing angle.
Structural color is studied in the field of thin-film optics. A layman's term that describes particularly the most ordered structural colors is iridescence.
Additional terms
- Hue: the color's location on the spectrum.
- Saturation: how "dense" or "intense" or "bright" a color is.
- Value: how light or dark a color is.
- Tint: a color made lighter by adding white.
- Shade: a color made darker by adding black.
Notes
- "Under well-lit viewing conditions (photopic vision), cones...are highly active and rods are inactive." Hirakawa, K, and Parks, TW, Chromatic adaptation and the white-balance problem,
- Jameson, K. A., Highnote, S. M., & Wasserman, L. M. (2001). Richer color experience in observers with multiple photopigment opsin genes. Psychonomic Bulletin and Review, 8(2), 244-261.
See also
- Metamerism
- Chromophore
- List of colors
- Qualia
- Color blindness
- Color temperature
- Color theory
- Color scheme
- Colors and emblems for parties
- Political color
- Color psychology
- Color Symbolism
- Synesthesia
- Goethe's Theory of Colors
- Kruithof curve
- The International Commission on Illumination defines colors and color spaces
- Thermochromics
- Tincture (heraldry). The colors in heraldry.
External links and sources
- COLOURlovers.com - Color Trends & Inspiration
- Comparative Article examining Goethean and Newtonian Color
- Kruithof curve citation
- Article by technical lighting manufacturer on rod/cone vision, with cites to literature
- Stanford Encyclopedia of Philosophy entry
- Why are things colored?
- Why Should Engineers and Scientists Be Worried About Color?
- Color, Contrast & Dimension in News Design
- Color Science
Template:Link FATemplate:Link FA
Categories: /nm
/nm
 /10 Hz
/10 Hz
 /10 cm
/10 cm
 /eV
/eV