This is an old revision of this page, as edited by ComplexRational (talk | contribs) at 13:27, 31 July 2019 (→Nuclide stability: A is isotopic mass). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 13:27, 31 July 2019 by ComplexRational (talk | contribs) (→Nuclide stability: A is isotopic mass)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) For the speech by Jimmy Carter, see Island of Stability (speech).
| Nuclear physics |
|---|
 |
| Models of the nucleus |
Nuclides' classification
|
| Nuclear stability |
| Radioactive decay |
| Nuclear fission |
| Capturing processes |
| High-energy processes |
|
Nucleosynthesis and nuclear astrophysics
|
| High-energy nuclear physics |
| Scientists |
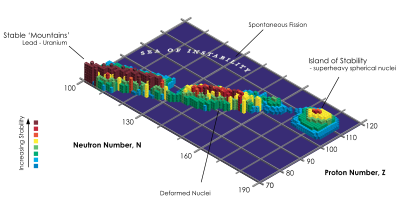
In nuclear physics, the island of stability is the prediction that a set of superheavy nuclides with magic numbers of protons and neutrons will temporarily reverse the trend of decreasing stability in elements heavier than uranium.
Various predictions have been made regarding the exact location of the island of stability, though it is generally thought to center near copernicium and flerovium isotopes (such as Cn, Cn, and Fl) approaching the predicted closed shell at neutron number N = 184. It is thought that the closed shell will confer additional stability towards fission, while also leading to longer half-lives towards alpha decay. While these effects are expected to be greatest near atomic number Z = 114 and N = 184, the region of increased stability is expected to encompass several neighboring elements, and there may also be additional islands of stability around heavier nuclei that are doubly magic (having magic numbers of both protons and neutrons). Estimates of the stability of the elements on the island are usually around a half-life of minutes or days; however, some estimates predict half-lives of millions of years.
Although the nuclear shell model predicting magic numbers has existed since the 1940s, the existence of long-lived superheavy nuclides has not been definitively demonstrated. Like the rest of the superheavy elements, the nuclides on the island of stability have never been found in nature; thus, they must be created artificially in a nuclear reaction to be studied. Scientists have not found a way to carry out such a reaction; it is likely that new types of reactions will be needed to populate nuclei near the center of the island. Nevertheless, the successful synthesis of superheavy elements up to oganesson in recent years demonstrates a slight stabilizing effect around elements 110–114 that may continue in unknown isotopes, supporting the existence of the island of stability.
Introduction

Nuclide stability
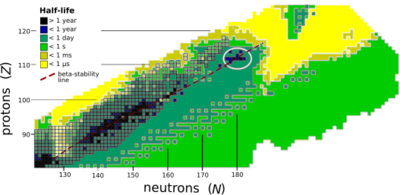
See also: Valley of stabilityThe composition of an atomic nucleus is determined by the number of protons Z and the number of neutrons N, which sum to an isotopic mass A. The atomic number Z determines the position of an element in the periodic table, but the more than 3000 nuclides are commonly represented in a chart with Z and N for its axes and the half-life for radioactive decay indicated for each unstable nuclide (see figure). 252 nuclides are thought to be stable (having never been observed to decay), and these follow a general trend in which the number of neutrons rises more rapidly than the number of protons. The last element in the periodic table that has a stable isotope is lead (Z = 82), with stability generally decreasing in heavier elements. The half-lives of nuclei also decrease when there is a lopsided neutron-proton ratio, such that the resulting nuclei have too few or too many neutrons to be stable.
The stability of a nucleus is determined by its binding energy, with higher binding energy conferring greater stability. The binding energy per nucleon increases with atomic number to a broad plateau around A = 60, then declines. If a nucleus can be split into two parts that have a lower total energy (a consequence of the mass defect resulting from greater binding energy), it is unstable. The nucleus can hold together for a finite time because there is a potential barrier opposing the split, but this barrier can be crossed by quantum tunnelling. The lower the barrier and the masses of the constituents, the greater the probability per unit time of a split.
Protons in a nucleus are bound together by the strong force, which counterbalances the Coulomb repulsion between positively charged protons. In heavier nuclei, larger numbers of neutrons are needed to reduce repulsion and confer additional stability. Even so, as physicists started to synthesize elements that are not found in nature, they found the stability decreased as the nuclei became heavier. Thus, they speculated that the periodic table might come to an end. The discoverers of plutonium (element 94) considered naming it "ultimium", thinking it was the last. Following the discoveries of heavier elements, of which some decayed in microseconds, it then seemed that element 108 might be the limit.
Magic numbers
The possible existence of superheavy elements with atomic numbers well beyond that of uranium had been suggested as far back as 1955 by John Archibald Wheeler, who is credited with the first usage of the term "superheavy element" in a 1958 paper published alongside Frederick Werner. However, this idea did not attract wide interest until a decade later, after improvements in the nuclear shell model. In this model, the atomic nucleus is built up in "shells", analogous to electron shells in atoms. Independently of each other, neutrons and protons have energy levels that are normally close together, but after a given shell is filled, it takes substantially more energy to start filling the next. Thus, the binding energy per nucleon reaches a local maximum and nuclei with filled shells are more stable than those without. This theory of a nuclear shell model originates in the 1930s, but it was not until 1949 that Maria Goeppert Mayer and J. Hans D. Jensen et al. independently devised the correct formulation. The numbers of nucleons for which shells are filled are called magic numbers, and magic numbers of 2, 8, 20, 28, 50, 82 and 126 have been observed for neutrons, and the next number is predicted to be 184. Protons share the first six of these magic numbers, and 126 has been predicted since the 1940s. Nuclides with a magic number of each are referred to as "doubly magic" and are more stable than nearby nuclides as a result of greater binding energies.
In the late 1960s, more sophisticated shell models by William Myers and Władysław Świątecki, and by Heines Meldner, taking into account Coulomb repulsion, changed the prediction for the next proton magic number from 126 to 114. Some Russian physicists argued for the existence of the doubly magic nuclide Fl (Z = 114, N = 184), rather than Ubh (Z = 126, N = 184) which was predicted to be doubly magic as early as 1957. Subsequently, estimates of the proton magic number have ranged from 114 to 126, and there is still no consensus. Myers and Świątecki appear to have coined the term "island of stability", and Glenn Seaborg, later a discoverer of many of the superheavy elements, quickly adopted the term and promoted it. Myers and Świątecki also proposed that some superheavy nuclei would be longer-lived as a consequence of a higher fission barrier. Further improvements in the nuclear shell model by Vilen Strutinsky led to the emergence of the macroscopic-microscopic method, taking into consideration both smooth trends characteristic of the liquid drop model and local fluctuations such as shell effects. This approach enabled Sven Nilsson et al., as well as other groups, to make the first detailed calculations of the stability of nuclei within the island.
Discoveries
| Element | Atomic number |
Most stable isotope |
Half-life | |
|---|---|---|---|---|
| Publications | NUBASE 2016 | |||
| Rutherfordium | 104 | Rf | 1.3 h | 2.5 h |
| Dubnium | 105 | Db | 1.2 d | 1.1 d |
| Seaborgium | 106 | Sg | 14 min | 5 min |
| Bohrium | 107 | Bh | 1 min | 3.8 min |
| Hassium | 108 | Hs | 9.7 s | 16 s |
| Meitnerium | 109 | Mt | 4.5 s | 7 s |
| Darmstadtium | 110 | Ds | 12.7 s | 14 s |
| Roentgenium | 111 | Rg | 1.7 min | 1.6 min |
| Copernicium | 112 | Cn | 28 s | 32 s |
| Nihonium | 113 | Nh | 9.5 s | 7 s |
| Flerovium | 114 | Fl | 1.9 s | 2.4 s |
| Moscovium | 115 | Mc | 650 ms | 410 ms |
| Livermorium | 116 | Lv | 57 ms | 80 ms |
| Tennessine | 117 | Ts | 51 ms | 70 ms |
| Oganesson | 118 | Og | 690 µs | 1.15 ms |

Interest in a possible island of stability grew throughout the 1960s, as some calculations suggested that it might contain nuclides with half-lives of billions of years. They were also predicted to be especially stable against spontaneous fission in spite of their high atomic mass. It was thought that if such elements exist and are sufficiently long-lived, there may be several novel applications as a consequence of their nuclear and chemical properties. These include use in particle accelerators as neutron sources, in nuclear weapons as a consequence of their probably low critical masses, and as nuclear fuel to power space missions. These speculations led many researchers to conduct searches for superheavy elements in the 1960s and 1970s, both in nature and through nucleosynthesis at particle accelerators.
During the 1970s, many searches for long-lived superheavy nuclei were conducted. Experiments aimed at synthesizing various elements ranging in atomic number from 110 to 127 were conducted at various laboratories around the world, though none were successful, indicating that such experiments may have been insufficiently sensitive if cross sections were low, or that any nuclei reachable via such fusion-evaporation reactions would be too short-lived for detection. More recent experiments reveal that this indeed may be the case. Similar searches in nature were also unsuccessful, suggesting that if superheavy elements do exist in nature, their abundance is less than 10 moles of superheavy elements per mole of ore. Despite these failures, new superheavy elements were being discovered every few years in various laboratories through light-ion bombardment and cold fusion reactions; rutherfordium, the first transactinide, was discovered in 1969, and copernicium, eight protons closer to the island of stability predicted at Z = 114, was reached by 1996. Even though the half-lives of these nuclei are very short (on the order of seconds), the very existence of elements heavier than rutherfordium is indicative of stabilizing effects thought to be caused by closed shells; a model not considering such effects would forbid the existence of these elements due to rapid spontaneous fission.
Flerovium, with the expected magic 114 protons, was first synthesized in 1998 by Yuri Oganessian et al. at the Joint Institute for Nuclear Research in Dubna, Russia. A single atom of element 114 was detected, with a lifetime of 30.4 seconds, and its decay products had half-lives measurable in minutes. This was seen as a "textbook example" of a decay chain characteristic of the island of stability, and provided strong evidence for the existence of the island of stability in this region. Even though the original 1998 chain was not observed again, and its assignment remains uncertain, further successful experiments in the next two decades led to the discovery of all elements up to oganesson, whose half-lives were found to exceed initially predicted values; these decay properties further support the presence of the island of stability. Although known nuclei still fall several neutrons short of N = 184 where maximum stability is expected (the most neutron-rich confirmed nuclei, Lv and Ts, only reach N = 177), and the exact location of the center of the island remains unknown, the trend of increasing stability closer to N = 184 has been demonstrated. For example, the isotope Cn, with eight more neutrons than Cn, has a half-life almost five orders of magnitude longer; this is expected to continue into unknown heavier isotopes.
Deformed nuclei
Studies from the early 1990s have shown that superheavy elements do not have perfectly spherical nuclei. A shell is considered stable when it is in a spherical form. A change in the shape of the nucleus changes the position of neutrons and protons in the shell; recent research indicates that large nuclei are deformed, causing magic numbers to shift or new magic numbers to appear. Current theoretical investigation indicates that in the region Z = 106–108 and N ≈ 160–164, nuclei may be more resistant to fission as a consequence of shell effects for deformed nuclei; thus, such superheavy nuclei would only undergo alpha decay. Hassium-270 is now believed to be a doubly magic deformed nucleus, with deformed magic numbers Z = 108 and N = 162. It has a half-life of 10 seconds. This is consistent with models that take into account the deformed nature of nuclei intermediate between the actinides and island of stability near N = 184, in which a stability "peninsula" emerges at deformed magic numbers Z = 108 and N = 162. Determination of the decay properties of neighboring hassium and seaborgium isotopes near N = 162 provides additional strong evidence for this region of relative stability in deformed nuclei. This also strongly suggests that the island of stability is not completely isolated from the region of stable nuclei, but rather that both regions are instead linked through a peninsula of relatively stable deformed nuclei.
Predicted decay properties
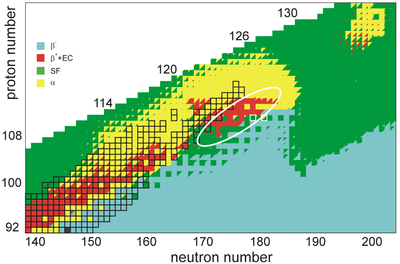
The half-lives of nuclei in the island of stability itself are unknown since none of the nuclides that would be "on the island" have been observed. Many physicists believe that the half-lives of these nuclei are relatively short, on the order of minutes or days. Some theoretical calculations indicate that their half-lives may be long, on the order of 100 years, or possibly as long as 10 years.
The shell closure at N = 184 is predicted to result in longer partial half-lives for alpha decay and spontaneous fission. It is believed that the shell closure will result in higher fission barriers for nuclei around Fl, strongly hindering fission and perhaps resulting in fission half-lives 30 orders of magnitude greater than those of nuclei unaffected by the shell closure. For example, the doubly magic nucleus Fl may have a spontaneous fission half-life on the order of 10 years; this is much longer than the 2.5 ms half-life of the known neutron-deficient isotope Fl (with N = 170) believed to demarcate the limit of stabilizing effects. Some undiscovered isotopes are predicted to undergo fission with still shorter half-lives, limiting the existence and possible observation of superheavy nuclei beyond the island of stability (namely for Z > 120 and N > 184; these nuclei may undergo alpha decay or spontaneous fission in microseconds or less). In the center of the island, there may be competition between alpha decay and spontaneous fission, though the exact ratio is strongly model dependent. The alpha-decay half-lives of 1700 nuclei with 100 ≤ Z ≤ 130 have been calculated in a quantum tunneling model with both experimental and theoretical alpha-decay Q-values, and are in agreement with observed half-lives for some of the heaviest isotopes. The longest lived isotopes are predicted to lie on the beta-stability line as well, for beta decay is predicted to compete with the other decay modes near the predicted center of the island, especially for isotopes of elements 111–115. The possible role of beta decay is highly uncertain, as isotopes of these elements (such as Fl and Mc) are predicted to have shorter partial half-lives for alpha decay; this would reduce competition and result in alpha decay remaining the dominant decay channel, unless additional stability towards alpha decay exists in superdeformed isomers of these nuclides. Considering all decay modes, various models indicate a shift of the center of the island (i.e. the longest-living nuclide) from Fl to a lower atomic number; these include 100-year half-lives for Cn and Cn, a 1000-year half-life for Cn, and a 300-year half-life for Ds; the latter two exactly at the N = 184 shell closure.
Even though these half-lives would be relatively long for superheavy elements, they are far too short for any such nuclides to exist primordially on Earth. It is thought that they may occur in cosmic rays at an abundance of 10 relative to lead, although instability of nuclei intermediate between primordial actinides (Th, U, and U) and the island of stability may inhibit their production in r-process nucleosynthesis. Various models suggest that spontaneous fission will be the dominant decay mode of nuclei with A > 280, and that neutron-induced or beta-delayed fission will become the primary reaction channels. As a result, beta decay towards the island of stability may only occur in a very narrow path or may be entirely blocked by fission, thus precluding the synthesis of nuclides within the island.
A possible stronger decay mode for the heaviest superheavy elements was shown to be cluster decay by Dorin N. Poenaru, R.A. Gherghescu, and Walter Greiner. This is predicted to have a greater impact above Z = 122, however, reducing its effect on the decay of isotopes near the center of the island, unless the center of the island is at a higher atomic number than predicted.
Synthesis problems
The manufacture of nuclei on the island of stability proves to be very difficult because the nuclei available as starting materials do not deliver the necessary sum of neutrons. Radioactive ion beams (such as S) in combination with actinide targets (such as Cm) may allow the production of more neutron rich nuclei nearer to the center of the island of stability, though such beams are not currently available in the required intensities to conduct such experiments. Several heavier isotopes such as Cm and Es may still be usable as targets, allowing the production of isotopes with one or two more neutrons than known isotopes, though the production of several milligrams of these rare isotopes to create a target is difficult. It may also be possible to probe alternative reaction channels in the same Ca-induced fusion-evaporation reactions that populate the most neutron-rich known isotopes, namely the pxn and αxn (emission of a proton or alpha particle, respectively, followed by several neutrons) channels; these may allow the synthesis of neutron-enriched isotopes of elements 111–117. Although the predicted cross sections are on the order of 1–900 fb, smaller than those in the xn (emission of neutrons only) channels, it may still be possible to generate otherwise unreachable isotopes of superheavy elements in these reactions. Some of these heavier isotopes may also undergo electron capture in addition to alpha decay with relatively long half-lives, decaying to nuclei such as Cn that are predicted to lie near the center of the island of stability, though this remains largely hypothetical as properties of superheavy nuclei near the beta-stability line remain unexplored.
It may also be possible to generate isotopes in the island of stability such as Fl in multi-nucleon transfer reactions in low-energy collisions of actinide nuclei (such as U and Cm). This inverse quasifission (partial fusion followed by fission, with a shift away from mass equilibrium in the products) mechanism may provide a path to the island of stability if shell effects around Z = 114 are sufficiently strong, though lighter elements such as nobelium and seaborgium (Z = 102–106) are predicted to have higher yields. Preliminary studies of the U + U and U + Cm transfer reactions have failed to produce elements heavier than mendelevium (Z = 101), though the increased yield in the latter reaction suggests that the use of even heavier targets such as Es (if available) may enable production of superheavy elements. A later study of the U + Th reaction found several unknown alpha decays that may possibly be attributed to new, neutron-rich isotopes of superheavy elements with 104 < Z < 116, though further research is required to unambiguously determine the atomic number of the products. This result strongly suggests that shell effects have a significant influence on cross sections, and that the island of stability may be reached in future experiments with transfer reactions.
Other islands of stability
Further shell closures beyond the main island of stability around Z = 114, N = 184 may give rise to additional islands of stability. It is thought that two significant islands may exist around heavier doubly magic nuclei; the first near 126 or 126 (with 216 or 228 neutrons) and the second near 164 or 164 (with 308 or 318 neutrons). These isotopes might be especially resistant to spontaneous fission and have alpha decay half-lives measurable in years, thus having comparable stability to elements in the vicinity of flerovium. Such a concept was proposed by Yuri Oganessian at the 235th national meeting of the American Chemical Society in 2008. However, substantially greater electromagnetic repulsion between protons in such heavy nuclei may reduce their stability, and possibly cause them to only briefly exist as unbound resonances. This may have the additional consequence of isolating these islands from the main chart of nuclides, as intermediate isotopes and perhaps elements in a "sea of instability" would rapidly undergo fission and essentially be nonexistent. It is also possible that beyond a region of relative stability around element 126, heavier nuclei would lie beyond a fission threshold given by the liquid drop model, thus rendering them nonexistent even in the vicinity of greater magic numbers.
As these elements are much heavier than any known elements, it is thought that a new, stronger particle accelerator will be needed for their synthesis.
It has also been posited that in the region beyond A > 300, an entire "continent of stability" consisting of a hypothetical phase of stable quark matter with only up and down quarks may exist. Such a form of matter is theorized to be a ground state of baryonic matter with a greater binding energy per baryon, favoring the decay of nuclear matter beyond this mass threshold into quark matter. If this state of matter exists, it could possibly be synthesized in the same fusion reactions leading to normal superheavy nuclei, and would be stabilized against fission as a consequence of its stronger binding that is enough to overcome Coulomb repulsion.
See also
Notes
- Different sources give different values for half-lives; the most recently published values in the literature and NUBASE are both listed below for reference.
- The unconfirmed Bh may have a longer half-life of 11.5 minutes.
- ^ For elements 109–118, the longest-lived known isotope is always the heaviest discovered thus far. This makes it seem likely that there are longer-lived undiscovered isotopes among the even heavier ones.
- The unconfirmed Mt may have a longer half-life of 1.1 minutes.
- The unconfirmed Rg may have a longer half-life of 10.7 minutes.
- The unconfirmed Fl may have a longer half-life of 19 seconds.
- The unconfirmed Og may have a longer half-life of 181 milliseconds.
References
- Zagrebaev, Valeriy (28 May 2012). Opportunities for synthesis of new superheavy nuclei (What really can be done within the next few years). 11th International Conference on Nucleus-Nucleus Collisions (NN2012). San Antonio, Texas, US. Archived from the original on 3 March 2016.
- Moskowitz, Clara (7 May 2014). "Superheavy Element 117 Points to Fabled "Island of Stability" on Periodic Table". Scientific American. Retrieved 20 April 2019.
- ^ Karpov, A.V.; Zagrebaev, V.I.; Palenzuela, Y.M.; Ruiz, L.F.; Greiner, W. (2012). "Decay properties and stability of the heaviest elements" (PDF). International Journal of Modern Physics E. 21 (2): 1250013–1–1250013–20. Bibcode:2012IJMPE..2150013K. doi:10.1142/S0218301312500139.
- ^ "Superheavy Element 114 Confirmed: A Stepping Stone to the Island of Stability". Berkeley Lab. September 24, 2009.
- ^ Oganessian, Y.T.; Rykaczewski, K. (2015). "A beachhead on the island of stability". Physics Today. 68 (8): 32–38. Bibcode:2015PhT....68h..32O. doi:10.1063/PT.3.2880.
- Podgorsak, Ervin B. (2016). Radiation physics for medical physicists (Third ed.). Springer. p. 512. ISBN 9783319253824.
- "Atomic structure". Australian Radiation Protection and Nuclear Safety Agency. Commonwealth of Australia. 26 April 2017. Retrieved 16 February 2019.
- Koura, H.; Katakura, J.; Tachibana, T.; Minato, F. (2015). "Chart of the Nuclides". Japan Atomic Energy Agency. Retrieved 12 April 2019.
- Podgorsak, Ervin B. (2016). Radiation physics for medical physicists (Third ed.). Springer. p. 33. ISBN 9783319253824.
- Blatt, John M.; Weisskopf, Victor F. (2012). Theoretical nuclear physics. Dover Publications. pp. 7–9. ISBN 9780486139500.
- ^ Sacks, Oliver (8 February 2004). "Greetings From the Island of Stability". The New York Times. Retrieved 16 February 2019.
- Hoffman 2000, p. 34
- ^ Hoffman 2000, p. 400
- Kragh, H. (2013). "Superheavy elements and the upper limit of the periodic table: early speculations". European Physical Journal H. 38 (3): 411–431. arXiv:1207.5946. doi:10.1140/epjh/e2012-30043-7.
- Nave, R. "Shell Model of Nucleus". HyperPhysics. Department of Physics and Astronomy, Georgia State University. Retrieved 22 January 2007.
- Caurier, E.; Martínez-Pinedo, G.; Nowacki, F.; Poves, A.; Zuker, A.P. (2005). "The shell model as a unified view of nuclear structure". Reviews of Modern Physics. 77 (2). arXiv:nucl-th/0402046. doi:10.1103/RevModPhys.77.427.
- ^ Oganessian, Yuri Ts.; Rykaczewski, Krzysztof P. (August 2015). "A beachhead on the island of stability". Physics Today. 68 (8): 32–38. doi:10.1063/PT.3.2880.
- Satake, M. (2010). Introduction to nuclear chemistry. Discovery Publishing House. p. 36. ISBN 9788171412778.
- Ebbing, Darrell; Gammon, Steven D. (2007). General chemistry (8th ed.). Houghton Mifflin. p. 858. ISBN 9780618738793.
- ^ Kragh, Helge (2018). From transuranic to superheavy elements : a story of dispute and creation. Springer. p. 22. ISBN 9783319758138.
- Dumé, Belle (15 June 2005). ""Magic" numbers remain magic". Physics World. IOP Publishing. Retrieved 17 February 2019.
- ^ Bemis, C.E.; Nix, J.R. (1977). "Superheavy elements - the quest in perspective" (PDF). Comments on Nuclear and Particle Physics. 7 (3): 65–78. ISSN 0010-2709.
- ^ Courtland, R. (10 February 2010). "Weight scale for atoms could map 'island of stability'". NewScientist. Retrieved 4 July 2019.
- Koura, H.; Chiba, S. (2013). "Single-Particle Levels of Spherical Nuclei in the Superheavy and Extremely Superheavy Mass Region". Journal of the Physical Society of Japan. 82: 014201. doi:10.7566/JPSJ.82.014201.
- Emsley 2011, p. 566
- Oganessian, Y.T.; Utyonkov, V.K. (2015). "Super-heavy element research". Reports on Progress in Physics. 78 (3): 036301. Bibcode:2015RPPh...78c6301O. doi:10.1088/0034-4885/78/3/036301. PMID 25746203.
{{cite journal}}: Invalid|ref=harv(help) - ^ Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
- ^ Utyonkov, V. K.; Brewer, N. T.; Oganessian, Yu. Ts.; Rykaczewski, K. P.; Abdullin, F. Sh.; Dimitriev, S. N.; Grzywacz, R. K.; Itkis, M. G.; Miernik, K.; Polyakov, A. N.; Roberto, J. B.; Sagaidak, R. N.; Shirokovsky, I. V.; Shumeiko, M. V.; Tsyganov, Yu. S.; Voinov, A. A.; Subbotin, V. G.; Sukhov, A. M.; Karpov, A. V.; Popeko, A. G.; Sabel'nikov, A. V.; Svirikhin, A. I.; Vostokin, G. K.; Hamilton, J. H.; Kovrinzhykh, N. D.; Schlattauer, L.; Stoyer, M. A.; Gan, Z.; Huang, W. X.; Ma, L. (30 January 2018). "Neutron-deficient superheavy nuclei obtained in the Pu+Ca reaction". Physical Review C. 97 (14320): 014320. Bibcode:2018PhRvC..97a4320U. doi:10.1103/PhysRevC.97.014320.
- ^ Hofmann, S.; Heinz, S.; Mann, R.; Maurer, J.; Münzenberg, G.; Antalic, S.; Barth, W.; Burkhard, H. G.; Dahl, L.; Eberhardt, K.; Grzywacz, R.; Hamilton, J. H.; Henderson, R. A.; Kenneally, J. M.; Kindler, B.; Kojouharov, I.; Lang, R.; Lommel, B.; Miernik, K.; Miller, D.; Moody, K. J.; Morita, K.; Nishio, K.; Popeko, A. G.; Roberto, J. B.; Runke, J.; Rykaczewski, K. P.; Saro, S.; Scheidenberger, C.; Schött, H. J.; Shaughnessy, D. A.; Stoyer, M. A.; Thörle-Popiesch, P.; Tinschert, K.; Trautmann, N.; Uusitalo, J.; Yeremin, A. V. (2016). "Review of even element super-heavy nuclei and search for element 120". The European Physical Journal A. 2016 (52). Bibcode:2016EPJA...52..180H. doi:10.1140/epja/i2016-16180-4.
- ^ Oganessian, Y. (2007). "Heaviest nuclei from Ca-induced reactions" (PDF). Journal of Physics G: Nuclear and Particle Physics. 34 (4): R165 – R242. Bibcode:2007JPhG...34R.165O. doi:10.1088/0954-3899/34/4/R01.
- ^ Lodhi, M.A.K., ed. (March 1978). Superheavy Elements: Proceedings of the International Symposium on Superheavy Elements. Lubbock, Texas: Pergamon Press. ISBN 978-0-08-022946-1.
- ^ Oganessian, Y. (2012). "Nuclei in the "Island of Stability" of Superheavy Elements". Journal of Physics: Conference Series. 337 (1): 012005. Bibcode:2012JPhCS.337a2005O. doi:10.1088/1742-6596/337/1/012005.
- ^ Ćwiok, S.; Heenen, P.-H.; Nazarewicz, W. (2005). "Shape coexistence and triaxiality in the superheavy nuclei" (PDF). Nature. 433 (7027): 705–709. Bibcode:2005Natur.433..705C. doi:10.1038/nature03336. PMID 15716943. Archived from the original (PDF) on 2010-06-23.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - Gsponer, A.; Hurni, J.-P. (2009). Fourth Generation Nuclear Weapons: The physical principles of thermonuclear explosives, inertial confinement fusion, and the quest for fourth generation nuclear weapons (3rd printing of the 7th ed.). pp. 129–133.
- Emsley 2011, p. 588
- ^ Karpov, A.; Zagrebaev, V.; Greiner, W. (2015). "Superheavy Nuclei: Which regions of nuclear map are accessible in the nearest studies?" (PDF). SHE-2015. Retrieved 30 October 2018.
- Hoffman 2000, p. 403
- ^ Möller, P. (2016). "The limits of the nuclear chart set by fission and alpha decay" (PDF). EPJ Web of Conferences. 131. 03002:1–8. Bibcode:2016EPJWC.13103002M. doi:10.1051/epjconf/201613103002.
- Oganessian, Yu. Ts.; et al. (1999). "Synthesis of Superheavy Nuclei in the Ca + Pu Reaction" (PDF). Physical Review Letters. 83 (16): 3154. Bibcode:1999PhRvL..83.3154O. doi:10.1103/PhysRevLett.83.3154.
- Hoffman 2000, p. 426
- Oganessian, Yu. Ts.; Abdullin, F. Sh.; Bailey, P. D.; Benker, D. E.; Bennett, M. E.; Dmitriev, S. N.; Ezold, J. G.; Hamilton, J. H.; Henderson, R. A. (2010). "Synthesis of a New Element with Atomic Number Z=117". Physical Review Letters. 104 (142502): 142502. Bibcode:2010PhRvL.104n2502O. doi:10.1103/PhysRevLett.104.142502. PMID 20481935.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Zagrebaev, Valeriy; Karpov, Alexander; Greiner, Walter (2013). "Future of superheavy element research: Which nuclei could be synthesized within the next few years?" (PDF). Journal of Physics: Conference Series. Vol. 420. IOP Science. pp. 1–15. Retrieved 20 August 2013.
{{cite conference}}: Unknown parameter|booktitle=ignored (|book-title=suggested) (help) - Ćwiok, S.; Nazarewicz, W.; Heenen, P.H. (1999). "Structure of Odd-N Superheavy Elements". Physical Review Letters. 83 (6): 1108–1111. Bibcode:1999PhRvL..83.1108C. doi:10.1103/PhysRevLett.83.1108.
- ^ Samanta, C.; Chowdhury, P. R.; Basu, D. N. (2007). "Predictions of alpha decay half lives of heavy and superheavy elements". Nuclear Physics A. 789 (1–4): 142–154. arXiv:nucl-th/0703086. Bibcode:2007NuPhA.789..142S. CiteSeerX 10.1.1.264.8177. doi:10.1016/j.nuclphysa.2007.04.001.
- ^ Chowdhury, P. R.; Samanta, C.; Basu, D. N. (2008). "Search for long lived heaviest nuclei beyond the valley of stability". Physical Review C. 77 (4): 044603. arXiv:0802.3837. Bibcode:2008PhRvC..77d4603C. doi:10.1103/PhysRevC.77.044603.
- ^ Chowdhury, P. R.; Samanta, C.; Basu, D. N. (2008). "Nuclear half-lives for α-radioactivity of elements with 100 ≤ Z ≤ 130". Atomic Data and Nuclear Data Tables. 94 (6): 781–806. arXiv:0802.4161. Bibcode:2008ADNDT..94..781C. doi:10.1016/j.adt.2008.01.003.
- Dvořák, J. (2007). Decay properties of nuclei close to Z = 108 and N = 162 (PhD thesis). Technische Universität München.
- Dvorak, J. (2006). "Doubly Magic Nucleus
108Hs
162". Physical Review Letters. 97 (24): 242501. Bibcode:2006PhRvL..97x2501D. doi:10.1103/PhysRevLett.97.242501. PMID 17280272.{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Möller, P.; Nix, J.R. (1998). "Stability and Production of Superheavy Nuclei". AIP Conference Proceedings. 425 (1): 75. arXiv:nucl-th/9709016. doi:10.1063/1.55136.
- ^ Koura, H. (2011). Decay modes and a limit of existence of nuclei in the superheavy mass region (PDF). 4th International Conference on the Chemistry and Physics of the Transactinide Elements. Retrieved 18 November 2018.
- Chowdhury, P. R.; Samanta, C.; Basu, D. N. (2006). "α decay half-lives of new superheavy elements". Physical Review C. 73 (1): 014612. arXiv:nucl-th/0507054. Bibcode:2006PhRvC..73a4612C. doi:10.1103/PhysRevC.73.014612.
- Chowdhury, P. R.; Basu, D. N.; Samanta, C. (2007). "α decay chains from element 113". Physical Review C. 75 (4): 047306. arXiv:0704.3927. Bibcode:2007PhRvC..75d7306C. doi:10.1103/PhysRevC.75.047306.
- Samanta, C.; Basu, D. N.; Chowdhury, P. R. (2007). "Quantum tunneling in 112 and its alpha-decay chain". Journal of the Physical Society of Japan. 76 (12): 124201. arXiv:0708.4355. Bibcode:2007JPSJ...76l4201S. doi:10.1143/JPSJ.76.124201.
- Sarriguren, P. (2019). "Microscopic calculations of weak decays in superheavy nuclei". Physical Review C. 100 (1): 014309. arXiv:1907.06877. doi:10.1103/PhysRevC.100.014309.
- Nilsson, S. G.; et al. (1969). "On the nuclear structure and stability of heavy and superheavy elements". Nuclear Physics A (Submitted manuscript). 131 (1): 1–66. Bibcode:1969NuPhA.131....1N. doi:10.1016/0375-9474(69)90809-4.
- Petermann, I; Langanke, K.; Martínez-Pinedo, G.; Panov, I.V; Reinhard, P.G.; Thielemann, F.K. (2012). "Have superheavy elements been produced in nature?". European Physical Journal A. 48 (122). arXiv:1207.3432. doi:10.1140/epja/i2012-12122-6.
- Poenaru, D. N.; Gherghescu, R. A.; Greiner, W. (2011). "Heavy-Particle Radioactivity of Superheavy Nuclei". Physical Review Letters. 107 (6): 062503. arXiv:1106.3271. Bibcode:2011PhRvL.107f2503P. doi:10.1103/PhysRevLett.107.062503. PMID 21902317.
- ^ Popeko, A.G. (2016). Perspectives of SHE research at Dubna. NUSTAR Annual Meeting 2016. Helmholtzzentrum für Schwerionenforschung, Darmstadt, Germany.
- Roberto, J.B. (2015). "Actinide Targets for Super-Heavy Element Research" (PDF). cyclotron.tamu.edu. Texas A & M University. Retrieved 30 October 2018.
- ^ Hong, J.; Adamian, G.G.; Antonenko, N.V. (2017). "Ways to produce new superheavy isotopes with Z = 111–117 in charged particle evaporation channels". Physics Letters B. 764: 42–48. Bibcode:2017PhLB..764...42H. doi:10.1016/j.physletb.2016.11.002.
- Siwek-Wilczyńska, K.; Cap, T.; Kowal, P. (2018). "How to produce new superheavy nuclei?". arXiv:1812.09522 .
- Sekizawa, K. (2019). "TDHF theory and its extensions for the multinucleon transfer reaction: A mini review". arXiv:1902.01616.
- Zagrebaev, V.; Greiner, W. (2008). "Synthesis of superheavy nuclei: A search for new production reactions". Physical Review C. 78 (3): 034610. arXiv:0807.2537. Bibcode:2008PhRvC..78c4610Z. doi:10.1103/PhysRevC.78.034610.
- Schädel, M. (2016). "Prospects of heavy and superheavy element production via inelastic nucleus-nucleus collisions – from U + U to O + Es" (PDF). EPJ Web of Conferences. 131: 04001–1—04001–9. doi:10.1051/epjconf/201613104001.
- Wuenschel, S.; Hagel, K.; Barbui, M.; Gauthier, J.; Cao, X.G.; Wada, R.; Kim, E.J.; Majka, Z.; Planeta, R.; Sosin, Z.; Wieloch, A.; Zelga, K.; Kowalski, S.; Schmidt, K.; Ma, C.; Zhang, G.; Natowitz, J.B. (2018). "An experimental survey of the production of alpha decaying heavy elements in the reactions of U + Th at 7.5-6.1 MeV/nucleon". Physical Review C. 97 (6): 064602–1—064602–12. arXiv:1802.03091. doi:10.1103/PhysRevC.97.064602.
- ^ Greiner, W. (2013). "Nuclei: superheavy-superneutronic-strange-and of antimatter" (PDF). Journal of Physics: Conference Series. 413 (1): 012002. Bibcode:2013JPhCS.413a2002G. doi:10.1088/1742-6596/413/1/012002.
- ^ Okunev, V.S. (2018). "About islands of stability and limiting mass of the atomic nuclei". IOP Conference Series: Materials Science and Engineering. 468: 012012–1—012012–13. doi:10.1088/1757-899X/468/1/012012.
- Grumann, J.; Mosel, U.; Fink, B.; Greiner, W. (1969). "Investigation of the stability of superheavy nuclei around Z = 114 and Z = 164". Zeitschrift für Physik. 228 (5): 371–386. Bibcode:1969ZPhy..228..371G. doi:10.1007/BF01406719.
- "Nuclear scientists eye future landfall on a second 'island of stability'". Eurekalert.org. 2008-04-06. Retrieved 2014-05-02.
- Holdom, B.; Ren, J.; Zhang, C. (2018). "Quark matter may not be strange". Physical Review Letters. 120 (1): 222001–1—222001–6. arXiv:1707.06610. doi:10.1103/PhysRevLett.120.222001.
Bibliography
- Emsley, John (2011). Nature's Building Blocks: An A-Z Guide to the Elements (New ed.). New York, NY: Oxford University Press. ISBN 978-0-19-960563-7.
{{cite book}}: Invalid|ref=harv(help) - Hoffman, Darleane C.; Ghiorso, Albert; Seaborg, Glenn T. (2000). The Transuranium People: The Inside Story. World Scientific. ISBN 978-1-78-326244-1.
External links
- Here be stability (Nature, August 2006, with JINR diagram of heavy nuclides and predicted IoS)
- Can superheavy elements (such as Z = 116 or 118) be formed in a supernova? Can we observe them? (2004 – "maybe")
- Second postcard from the island of stability (2001; nuclides with 116 protons and mass 292
- First postcard from the island of nuclear stability (1999; first few Z = 114 atoms)
- NOVA: Island of Stability (2006; 13 m TV segment, with transcript)