| |
| Names | |
|---|---|
| Other names Butane-1,2,3,4-tetracarboxylic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H10O8 |
| Molar mass | 234.160 g·mol |
| Appearance | White solid |
| Melting point | 236 °C (457 °F; 509 K) 246 ºC for meso 227-230 ºC for (R,R) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
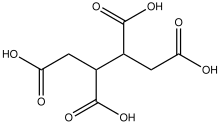
1,2,3,4-Butanetetracarboxylic acid is an organic compound with the formula HO2CCH2CH(CO2H)CH(CO2H)CH2CO2H. It is one of the simplest stable tetracarboxylic acids. The compound exists as two diastereomers, meso and the (R,R)/(S,S) pair. All are white solids. The compound is produced by oxidation of tetrahydrophthalic anhydride.
Uses and reactions
Among the several possible uses, it has been repeatedly investigated in the textile industry, e.g., for permanent press clothing. As expected for a polycarboxylate, it binds zinc to afford coordination polymers.
It forms a dianhydride (RN 4534-73-0), which consists of two succinic anhydride-like rings.
References
- ^ Nagao, R.; Marumo, F.; Saito, Y.; Asahara, T. (1971). "The Crystal Structure of Butane-1,2,3,4-tetracarboxylic Dianhydride". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 27 (3): 569–572. Bibcode:1971AcCrB..27..569N. doi:10.1107/s0567740871002577.
- Eltahlawy, K.; Elbendary, M.; Elhendawy, A.; Hudson, S. (2005). "The Antimicrobial Activity of Cotton Fabrics Treated with Different Crosslinking Agents and Chitosan". Carbohydrate Polymers. 60 (4): 421–430. doi:10.1016/j.carbpol.2005.02.019.
- Welch, Clark M. (1988). "Tetracarboxylic Acids as Formaldehyde-Free Durable Press Finishing Agents". Textile Research Journal. 58 (8): 480–486. doi:10.1177/004051758805800809.
- Wei, Guo-Hua; Yang, Jin; Ma, Jian-Fang; Liu, Ying-Ying; Li, Shun-Li; Zhang, Lai-Ping (2008). "Syntheses, Structures and Luminescent Properties of Zinc(II) and Cadmium(II) Coordination Complexes Based on New Bis(imidazolyl)ether and Different Carboxylate Ligands". Dalton Transactions (23): 3080–3092. doi:10.1039/b716657e. PMID 18521450.