| |
| Names | |
|---|---|
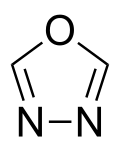
| Preferred IUPAC name 1,3,4-Oxadiazole | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C2H2N2O |
| Molar mass | 70.051 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
1,3,4-Oxadiazole is a nitrogen and oxygen containing heterocycle, and one of the four isomers of oxadiazole.
Derivatives
1,3,4-Oxadiazole itself is not commonly used in organic chemistry, but many of its derivatives are important. For example, raltegravir is an HIV drug which contains an 1,3,4-oxadiazole ring. Other pharmaceutical drugs containing the 1,3,4-oxadiazole ring include fenadiazole, zibotentan, and tiodazosin.
1,3,4-Oxadiazole derivatives can be synthesized in a variety of ways. One pathway is from oxidation of tetrazoles in the presence of aldehydes. Similarly, the reaction of tetrazoles with acyl chlorides provides oxadiazoles. Both methods involve the release of N2.
See also
- Furazan (1,2,5-oxadiazole)
References
- PubChem. "1,3,4-Oxadiazole". pubchem.ncbi.nlm.nih.gov. National Center for Biotechnology Information, U.S. National Library of Medicine. Retrieved 2019-07-22.
- Bala, Suman; Kamboj, Sunil; Kajal, Anu; Saini, Vipin; Prasad, Deo Nanadan (2014). "1,3,4-Oxadiazole Derivatives: Synthesis, Characterization, Antimicrobial Potential, and Computational Studies". BioMed Research International. 2014: 172791. doi:10.1155/2014/172791. PMC 4131560. PMID 25147788.
- "1,3,4-Oxadiazole synthesis". www.organic-chemistry.org. Retrieved 11 November 2018.
- Wang, Liang; Cao, Jing; Chen, Qun; He, Mingyang (17 April 2015). "One-Pot Synthesis of 2,5-Diaryl 1,3,4-Oxadiazoles via Di-tert-butyl Peroxide Promoted Acylation of Aryl Tetrazoles with Aldehydes". The Journal of Organic Chemistry. 80 (9): 4743–4748. doi:10.1021/acs.joc.5b00207. PMID 25860162.
- Wong, Michael Y.; Krotkus, Simonas; Copley, Graeme; Li, Wenbo; Murawski, Caroline; Hall, David; Hedley, Gordon J.; Jaricot, Marie; Cordes, David B.; Slawin, Alexandra M. Z.; Olivier, Yoann; Beljonne, David; Muccioli, Luca; Moral, Monica; Sancho-Garcia, Juan-Carlos; Gather, Malte C.; Samuel, Ifor D. W.; Zysman-Colman, Eli (7 September 2018). "Deep-Blue Oxadiazole-Containing Thermally Activated Delayed Fluorescence Emitters for Organic Light-Emitting Diodes". ACS Applied Materials & Interfaces. 10 (39): 33360–33372. doi:10.1021/acsami.8b11136. hdl:10023/18433. PMID 30192504.