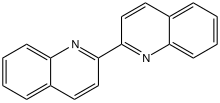
| |
| Names | |
|---|---|
| Preferred IUPAC name 2,2′-Biquinoline | |
| Other names 2,2′-Biquinolyl; 2-Quinolin-2-ylquinoline; Cuproin | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.003.961 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C18H12N2 |
| Molar mass | 256.308 g·mol |
| Appearance | White solid |
| Density | 1.374 g/cm |
| Melting point | 194.5 °C (382.1 °F; 467.6 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
2,2′-Biquinoline is an organic compound with the formula (C9H6N)2. It is one of several biquinolines. It is prepared by reductive coupling of 2-chloroquinoline. It is a colorimetric indicator for organolithium compounds.
Ligand properties

2,2′-Biquinoline is a bidentate ligand. Unlike the related complexes of 2,2′-bipyridine, the metal does not typically occupy the plane of the biquinoline.
References
- Folting, K.; Merritt, L. L. (1977). "2,2'-Biquinolyl". Acta Crystallographica Section B. 33 (11): 3540–3542. doi:10.1107/S0567740877011406.
- Nelson, Todd D.; Crouch, R. David (2004). "Cu, Ni, and Pd Mediated Homocoupling Reactions in Biaryl Syntheses: The Ullmann Reaction". Organic Reactions. 15: 265–555. doi:10.1002/0471264180.or063.03. ISBN 0471264180.
- Watson, Spencer Charles; Eastham, Jerome F. (1967). "Colored Indicators for Simple Direct Titration of Magnesium and Lithium Reagents". Journal of Organometallic Chemistry. 9: 165–8. doi:10.1016/S0022-328X(00)92418-5.
- Ha, Kwang (2012). "(2,2′-Biquinoline-κ-N,N′)dibromidopalladium(II)". Acta Crystallographica Section E. 68 (5): m618. doi:10.1107/S1600536812015425. PMC 3344352. PMID 22590118.