| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.008.421 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
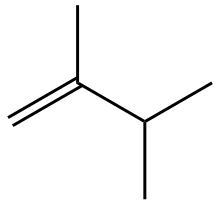
| Chemical formula | C6H12 |
| Molar mass | 84.162 g·mol |
| Appearance | colorless liquid |
| Density | 0.68 g/cm |
| Boiling point | 55 °C (131 °F; 328 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H203, H225 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P264+P265, P271, P280, P301+P316, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P319, P321, P331, P332+P317, P337+P317, P362+P364, P370+P378, P403+P233, P403+P235, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
2,3-Dimethyl-1-butene is an organic compound with the formula CH2=C(CH3)CH(CH3)2. Like the other isomers of dimethylbutene, it is a colorless liquid. Together with 2,3-dimethyl-2-butene it can be produced by dimerization of propylene. It is a precursor to the commercial fragrance tonalide.
References
- Olivier-Bourbigou, H.; Breuil, P. A. R.; Magna, L.; Michel, T.; Espada Pastor, M. Fernandez; Delcroix, D. (2020). "Nickel Catalyzed Olefin Oligomerization and Dimerization" (PDF). Chemical Reviews. 120 (15): 7919–7983. doi:10.1021/acs.chemrev.0c00076. PMID 32786672. S2CID 221124789.