| |

| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.019.010 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C6H14O2 |
| Molar mass | 118.176 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
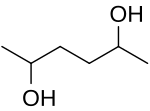
2,5-Hexanediol is an organic compound with the formula CH3CH(OH)CH2CH2CH(OH)CH3. It is both a glycol and a secondary alcohol. It is a colorless water-soluble viscous liquid. The chemical properties are well understood and have been extensively reported and studied. It has the IUPAC name of hexane-2,5-diol and the CAS Registry Number CAS 2935-44-6.
Other names
- (2R,5R)-2,5-hexanediol
- 2,5-Dihydroxyhexane
- Diisopropanol
- Hexan-2,5-diol
- -2,5-hexanediol
- hexane-2,5-diol
Manufacture
One common method of manufacture of the compound is from yeast. Another method involves the reduction of acetonylacetone. The material has two chiral carbons and thus has a number of enantiomers. Processes have been researched and developed to produce enantiopure products and by a continuous process. Some synthesis has been carried out from keto hexanoates.
Uses
One of the uses of the material is to synthesize polyesters. and also fine chemicals.
Toxicity
The toxicity of the material has been studied and is reasonably well understood. It can affect the eyes and has some neurotoxic effects.
References
- PubChem. "2,5-Hexanediol". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-01-04.
- "hexane-2,5-diol properties". materials.springer.com. Retrieved 2024-01-04.
- Peter Werle; Marcus Morawietz; Stefan Lundmark; Kent Sörensen; Esko Karvinen; Juha Lehtonen (2008). "Alcohols, Polyhydric". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_305.pub2. ISBN 978-3527306732.
- "2,5-Hexanediol (CAS 2935-44-6)". Cheméo. Retrieved 2024-01-04.
- "2,5-Hexanediol (CAS 2935-44-6)". Cheméo. Retrieved 2024-01-04.
- PubChem. "2,5-Hexanediol". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-01-04.
- "2,5-Hexanediol". nist.gov.
- Xiao, Meitian; Ye, Jing; Zhang, Yawu; Huang, Yayan (2009-06-01). "Reaction Characteristics of Asymmetric Synthesis of (2S,5S)-2,5-Hexanediol Catalyzed with Baker's Yeast Number 6". Chinese Journal of Chemical Engineering. 17 (3): 493–499. doi:10.1016/S1004-9541(08)60236-0. ISSN 1004-9541.
- Lieser, Joan K. (July 1983). "A Simple Synthesis of (S, S)-2, 5-Hexanediol". Synthetic Communications. 13 (9): 765–767. doi:10.1080/00397918308063707. ISSN 0039-7911.
- Haberland, Jürgen; Hummel, Werner; Daussmann, Thomas; Liese, Andreas (2002-07-01). "New Continuous Production Process for Enantiopure (2 R ,5 R )-Hexanediol". Organic Process Research & Development. 6 (4): 458–462. doi:10.1021/op020023t. ISSN 1083-6160.
- Schroer, Kirsten; Lütz, Stephan (2009-11-20). "A Continuously Operated Bimembrane Reactor Process for the Biocatalytic Production of (2 R ,5 R )-Hexanediol". Organic Process Research & Development. 13 (6): 1202–1205. doi:10.1021/op9001643. ISSN 1083-6160.
- Rudloff, E. von (1958-03-01). "SYNTHESIS OF SOME HEXANEDIOLS". Canadian Journal of Chemistry. 36 (3): 486–491. doi:10.1139/v58-069. ISSN 0008-4042.
- O'Malley, James J.; Stauffer, Walter J. (April 1974). "Synthesis and characterization of isomeric polyesters based on sebacic acid and hexanediols". Journal of Polymer Science: Polymer Chemistry Edition. 12 (4): 865–874. Bibcode:1974JPoSA..12..865O. doi:10.1002/pol.1974.170120416. ISSN 0360-6376.
- Herrmann, Wolfgang; Cornils, Boy; Zanthoff, Horst; Xu, Jian-He, eds. (2019-04-08). Catalysis from A to Z: A Concise Encyclopedia (1 ed.). Wiley. doi:10.1002/9783527809080.cataz09294. ISBN 978-3-527-34311-9. S2CID 219100145.
- Kannan, K.; Singh, K. P.; Goel, S. K.; Shanker, Ravi (1985-02-01). "Effect of 2,5-hexanediol on immunocompetence of mice". Environmental Research. 36 (1): 14–25. Bibcode:1985ER.....36...14K. doi:10.1016/0013-9351(85)90003-9. ISSN 0013-9351. PMID 3967636.
- Abou-Donia, Mohamed B.; Makkawy, H. M.; Campbell, Gerald M. (January 1985). "Pattern of neurotoxicity of n -hexane, methyl n -butyl ketone, 2,5-hexanediol, and 2,5-hexanedione alone and in combination with O -ethyl O -4-nitrophenyl phenylphosphonothioate in hens". Journal of Toxicology and Environmental Health. 16 (1): 85–100. doi:10.1080/15287398509530721. ISSN 0098-4108. PMID 4068058.
- Abou-Donia, Mohamed B.; Makkawy, Hany-Anwar M.; Graham, Doyle G. (1982-03-15). "The relative neurotoxicities of n-hexane, methyl n-butyl ketone, 2,5-hexanediol, and 2,5-hexanedione following oral or intraperitoneal administration in hens". Toxicology and Applied Pharmacology. 62 (3): 369–389. doi:10.1016/0041-008X(82)90139-9. ISSN 0041-008X. PMID 7071856.
- Eben, Anneliese; Flucke, Winfried; Mihail, Florin; Thyssen, Jürgen; Kimmerle, Georg (1979-06-01). "Toxicological and metabolic studies of methyl n-butylketone, 2,5-hexanedione, and 2,5-hexanediol in male rats". Ecotoxicology and Environmental Safety. 3 (2): 204–217. doi:10.1016/0147-6513(79)90012-5. ISSN 0147-6513. PMID 540560.
- Jones, H. B.; Cavanagh, J. B. (1982-12-01). "Recovery from 2,5-hexanediol intoxication of the retinotectal tract of the rat". Acta Neuropathologica. 58 (4): 286–290. doi:10.1007/BF00688611. ISSN 1432-0533. PMID 6891552. S2CID 39467729.