| This article may be too technical for most readers to understand. Please help improve it to make it understandable to non-experts, without removing the technical details. (January 2023) (Learn how and when to remove this message) |
| |
| Names | |
|---|---|
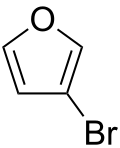
| Preferred IUPAC name 3-Bromofuran | |
| Other names 3-Furyl bromide; β-Bromofuran | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.040.662 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H3BrO |
| Molar mass | 146.971 g·mol |
| Density | 1.6606 @20 °C |
| Boiling point | 102.5 to 102.6 °C (216.5 to 216.7 °F; 375.6 to 375.8 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
3-Bromofuran is a colorless, organic compound with the molecular formula C4H3BrO. A versatile intermediate product for synthesizing more complex compounds, it used in the syntheses of a variety of economically important drugs.
A liquid at room temperature, it has a similar boiling point to water (102.5-102.6 °C), but with a significantly higher density (1.6606 g/cm3 at 20 °C). While colorless when pure, it can appear light yellow when minor impurities are present. It is usually stabilized by calcium carbonate.
Synthesis
3-Bromofuran was obtained in minor amounts in 1887 as a by-product in a reaction of 3-bromofuroic acid with calcium hydroxide. Four decades later, it was prepared deliberately and in higher yield. 3-bromofuran has since also been prepared from 3,4-dibromofuran via ortho-metalation with butyllithium in good yield. A synthesis of 3-bromofuran is due to Fechtel who prepared this compound via a Diels Alder-bromination-reverse Diels Alder sequence.
Applications
3-Bromofuran is a useful starting material for 3-substituted furans, a structural motif widespread in chemotherapy agents, HIV drugs, type 2 diabetes treatments, drugs for osteoporosis and experimental drugs for Alzheimer's disease. For example, the total synthesis of (+)Cacospongionolide B, a sesterterpene with anti-inflammatory properties, has been accomplished using 3-bromofuran as a starting compound. It was also used to synthesize Rosefuran, a constituent chemical of the odor of the rose and an insect sex attractant. 3-bromofuran was reacted with 3,3-dimethylallyl bromide and lithium diisopropylamide, followed by reaction at with iodomethane and N-butyllithium.
The total synthesis of (−)-neothiobinupharidine, a bioactive alkaloid isolated from Nuphar pumila (the small yellow pond-lily) was accomplished in eight steps employing two moles of 3-bromofuran. Similarly, one of the steps of the total synthesis of Salvinorin A, the primary hallucinogenic compound in Salvia divinorum, a Mexican plant used by Mazatec shamans, used 3-bromofuran as a reactant.
References
- ^ A. F. Shepard; Winslow, N. R.; Johnson, John R. (1930). "The simple halogen derivatives of furan". J. Am. Chem. Soc. 52 (9): 2083–2090. doi:10.1021/ja01368a057.
- Conzoneri; Oliveri (1887). Gazz. Chim. Ital. 17: 43.
{{cite journal}}: CS1 maint: untitled periodical (link) - Carlos Alvarez-Ibarra; Quiroga, Maria L.; Toledano, Emilio (1996). "Synthesis of polysubstituted 3-thiofurans by regiospecific mono-ipso-substitution and ortho-metallation from 3,4-dibromofuran". Tetrahedron. 52 (11): 4065–4078. doi:10.1016/s0040-4020(96)00069-5.
- Guenter Fechtel, "Preparation of furan and cyclopentadiene derivatives as biocides and drug intermediates" East Ger. Patent 246,107 (1987)
- Han-Zhong Zhang; Kasibhatla, Shailaja; Kuemmerle, Jared (2005). "Discovery and Structure-Activity Relationship of 3-Aryl-5-aryl-1,2,4-oxadiazoles as a New Series of Apoptosis Inducers and Potential Anticancer Agents". Journal of Medicinal Chemistry. 48 (16): 5215–5223. doi:10.1021/jm050292k. PMID 16078840.
- Susan E. Hagen; Domagla, John; Gajda, Christopher (2001). "4-Hydroxy-5,6-dihydropyrones as inhibitors of HIV protease: the effect of heterocyclic substituents at C-6 on antiviral potency and pharmacokinetic parameters". Journal of Medicinal Chemistry. 44 (14): 2319–2332. doi:10.1021/jm0003844. PMID 11428926.
- Qun Dang; Brown, Brian S.; Liu, Yan (2009). "Fructose-1,6-bisphosphatase Inhibitors. 1. Purine Phosphonic Acids as Novel AMP Mimics". Journal of Medicinal Chemistry. 52 (14): 2880–2898. doi:10.1021/jm900078f. PMID 19348494.
- Zhi-Cai Shi, et al., " Preparation of 4--(S)-phenylalanine derivative tryptophan hydroxylase inhibitors for treating osteoporosis ", US PCT Int. Appl. (2010), WO 2010065333 A1 20100610.
- Zhi-Cai Shi, et al., " Preparation of a (((1,2,4-oxadiazolyl)phenyl)morpholino)pyrimidin-4-one compound as a therapeutic tau protein kinase inhibitor ", PCT Int. Appl. (2009), WO 2009035162 A1 20090319.
- Inmaculada Posadas; De Rosa, Salvatore; Terencio, M Carmen (2003). "Cacospongionolide B suppresses the expression of inflammatory enzymes and tumour necrosis factor-α by inhibiting nuclear factor-κB activation". Br J Pharmacol. 138 (8): 1571–1579. doi:10.1038/sj.bjp.0705189. PMC 1573800. PMID 12721113.
- Motoko Oshida; Ono, Misaki; Nakazaki, Atsuo (2010). "Total synthesis of (+)-cacospongionolide B". Heterocycles. 80 (1): 313–328. doi:10.3987/com-09-s(s)17.
- Peter Weyerstahl; Schenk, Anja; Marschall, Helga (1995). "Structure-odor correlation. Part XXI. Olfactory properties and convenient synthesis of furans and thiophenes related to rosefuran and perillene and their isomers". Liebigs Annalen. 6 (10): 1849–1853. doi:10.1002/jlac.1995199510259.
- Daniel J. Jansen; Shenvi, Ryan A. (2013). "Synthesis of (−)-Neothiobinupharidine". Journal of the American Chemical Society. 135 (4): 1209–1212. doi:10.1021/ja310778t. PMID 23298203.
- Hisahiro Hagiwara; Suka, Yuhki; Nojima, Takashi; Suzuki, Toshio (2005). "Second-generation synthesis of salvinorin A". Tetrahedron. 65 (25): 4820–4825. doi:10.1016/j.tet.2009.04.053.