| |
| Names | |
|---|---|
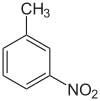
| Preferred IUPAC name 1-Methyl-3-nitrobenzene | |
| Other names m-Nitrotoluene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.480 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H7NO2 |
| Molar mass | 137.138 g·mol |
| Appearance | yellow liquid |
| Odor | mild, aromatic |
| Density | 1.1581 g·cm @ 20°C |
| Melting point | 15.5 °C (59.9 °F; 288.6 K) |
| Boiling point | 232 °C (450 °F; 505 K) |
| Solubility in water | 0.05% (20°C) |
| Vapor pressure | 0.1 mmHg (20°C) |
| Magnetic susceptibility (χ) | -72.71·10 cm/mol |
| Hazards | |
| Flash point | 106 °C; 223 °F; 379 K |
| Explosive limits | 1.6%-? |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | TWA 5 ppm (30 mg/m) |
| REL (Recommended) | TWA 2 ppm (11 mg/m) |
| IDLH (Immediate danger) | 200 ppm |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
3-Nitrotoluene or meta-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is one of three isomers of nitrotoluene. A yellow liquid, it is used in the manufacture of meta-toluidine, which is an intermediate in the production of various dyes.
Synthesis and reactions
It is made by nitrating toluene by conventional mixed acid (acetyl nitrate doesn't produce it): this reaction mainly affords a 2:1 mixture of 2-nitro and 4-nitro isomers, but after removal of the 2-isomer, the 3-nitrotoluene can be purified by distillation. It is a precursor to toluidine, which is used in producing azo dyes.
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0463". National Institute for Occupational Safety and Health (NIOSH).
- ^ Lide DR, ed. (2004). CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data (85 ed.). Boca Ratan Florida: CRC Press. ISBN 0-8493-0485-7.
- ^ Gerald Booth (2007). "Nitro Compounds, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411. ISBN 978-3527306732.
- Amé Pictet; Eug. Khotinsky (January 1907). "Über Acetylnitrat". Berichte der Deutschen Chemischen Gesellschaft (in German). 40 (1): 1163–1166. doi:10.1002/CBER.190704001172. ISSN 0365-9496. Wikidata Q61714426.
External links
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |