| |
| |
| Names | |
|---|---|
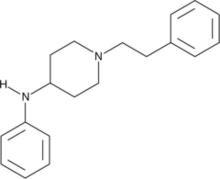
| Preferred IUPAC name N-Phenyl-1-(2-phenylethyl)piperidin-4-amine | |
| Other names desproprionyl fentanyl; 4-anilino-N-phenethylpiperidine; 4-ANPP; ANPP | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | ANPP |
| ChemSpider | |
| ECHA InfoCard | 100.169.974 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C19H24N2 |
| Molar mass | 280.415 g·mol |
| Legal status |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
4-ANPP, also known as 4-anilino-N-phenethylpiperidine (4-ANPP), 4-aminophenyl-1-phenethylpiperidine, or despropionyl fentanyl, is a direct precursor to fentanyl and acetylfentanyl. It is commonly found as a contaminant in samples of drugs containing fentanyl, which may include samples represented by the supplier as heroin or other opioids. It is not psychoactive and is present only as a result of improper chemical purification.
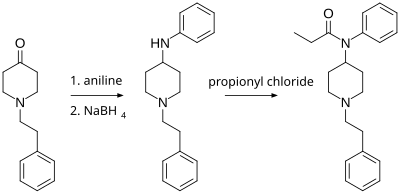
4-ANPP is useful in the synthesis of pharmaceuticals, primarily fentanyl and related analogs. Paul Janssen (founder of Janssen Pharmaceutica) first synthesized fentanyl in 1960 using a similar method, with Benzylfentanyl as an intermediate. The following synthesis, developed by an individual under the pseudonym of Siegfried, involves the reductive amination of N-phenethyl-4-piperidinone (NPP) with aniline to make to 4-ANPP. This product is reacted with propionyl chloride or acetyl chloride to form either fentanyl or acetylfentanyl.
See also
References
- "Red list". www.incb.org. Retrieved 2021-02-20.
- Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-15.
- "4-ANPP". www.caymanchem.com. Retrieved 2021-02-19.
- DrugsData.org. "DrugsData.org: Results : Lab Test Results for Fentanyl". www.drugsdata.org. Retrieved 2021-02-19.
- Schulz W. "Fentanyl". List of Top Pharmaceuticals. Chemical & Engineering News.