| |
| Names | |
|---|---|
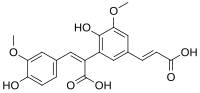
| Preferred IUPAC name (2E)-2-{5--2-hydroxy-3-methoxyphenyl}-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid | |
| Other names
8,5′-DiFA Ferulic acid 8-5-dehydrodimer 8,5′-diFA (open form) 8,5-DiFA 5-8′-Dehydrodiferulic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H18O8 |
| Molar mass | 386.356 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
8,5′-Diferulic acid is a non cyclic type of diferulic acid. It is the predominant diferulic acid in sugar beet pulp. It is also found in barley, in maize bran and rye. 8,5′-Diferulic acid has also been identified to be covalently linked to carbohydrate moieties of the arabinogalactan-protein fraction of gum arabic.
See also
References
- "8,5'-Diferulic acid | Chemical Substance Information | J-GLOBAL". jglobal.jst.go.jp.
- Micard, V.; Grabber, J.H.; Ralph, J.; Renard, C.M.G.C.; Thibault, J.-F. (1997). "Dehydrodiferulic acids from sugar-beet pulp". Phytochemistry. 44 (7): 1365–1368. Bibcode:1997PChem..44.1365M. doi:10.1016/S0031-9422(96)00699-1.
- Hernanz, D; Nuñez, V; Sancho, AI; Faulds, CB; Williamson, G; Bartolomé, B; Gómez-Cordovés, C (2001). "Hydroxycinnamic acids and ferulic acid dehydrodimers in barley and processed barley". Journal of Agricultural and Food Chemistry. 49 (10): 4884–8. Bibcode:2001JAFC...49.4884H. doi:10.1021/jf010530u. PMID 11600039.
- Bunzel, M; Funk, C; Steinhart, H (2004). "Semipreparative isolation of dehydrodiferulic and dehydrotriferulic acids as standard substances from maize bran". Journal of Separation Science. 27 (13): 1080–6. doi:10.1002/jssc.200301703. PMID 15495409.
- Andreasen, MF; Christensen, LP; Meyer, AS; Hansen, A (2000). "Content of phenolic acids and ferulic acid dehydrodimers in 17 rye (Secale cereale L.) varieties". Journal of Agricultural and Food Chemistry. 48 (7): 2837–42. Bibcode:2000JAFC...48.2837A. doi:10.1021/jf991266w. PMID 11032481.
- Renard, D; Lavenant-Gourgeon, L; Ralet, MC; Sanchez, C (2006). "Acacia senegal gum: Continuum of molecular species differing by their protein to sugar ratio, molecular weight, and charges". Biomacromolecules. 7 (9): 2637–49. doi:10.1021/bm060145j. PMID 16961328.
External links
| Types of hydroxycinnamic acids | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aglycones |
| ||||||||||||||||
| Esters |
| ||||||||||||||||
| Oligomeric forms |
| ||||||||||||||||
| Conjugates with coenzyme A (CoA) | |||||||||||||||||