 | |
| Clinical data | |
|---|---|
| Trade names | Amenalief |
| Other names | ASP-2151, ASP2151 |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
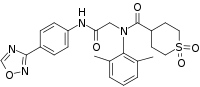
| Formula | C24H26N4O5S |
| Molar mass | 482.56 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Amenamevir (trade name Amenalief) is an antiviral drug used for the treatment of shingles (herpes zoster).
It acts as an inhibitor of the zoster virus's helicase–primase complex. Amenamevir was approved in Japan for the treatment of shingles in 2017.
See also
References
- Kawashima M, Nemoto O, Honda M, Watanabe D, Nakayama J, Imafuku S, et al. (November 2017). "Amenamevir, a novel helicase-primase inhibitor, for treatment of herpes zoster: A randomized, double-blind, valaciclovir-controlled phase 3 study". The Journal of Dermatology. 44 (11): 1219–1227. doi:10.1111/1346-8138.13948. PMC 5697646. PMID 28681394.
- Yajima M, Yamada H, Takemoto M, Daikoku T, Yoshida Y, Long T, et al. (March 2017). "Profile of anti-herpetic action of ASP2151 (amenamevir) as a helicase-primase inhibitor". Antiviral Research. 139: 95–101. doi:10.1016/j.antiviral.2016.12.008. PMID 28027917. S2CID 43813287.
- "Maruho Receives Manufacturing and Marketing Approval for Anti-Herpes Virus Agent "Amenalief® Tab. 200mg" in Japan" (Press release). evaluategroup.com. July 3, 2017.
| DNA virus antivirals (primarily J05, also S01AD and D06BB) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baltimore I |
| ||||||||||||||||||||
| Hepatitis B (VII) | |||||||||||||||||||||
| Multiple/general |
| ||||||||||||||||||||
| |||||||||||||||||||||
This antiinfective drug article is a stub. You can help Misplaced Pages by expanding it. |