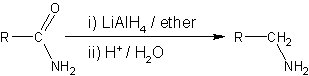Amide reduction is a reaction in organic synthesis where an amide is reduced to either an amine or an aldehyde functional group.
Catalytic hydrogenation
Catalytic hydrogenation can be used to reduce amides to amines; however, the process often requires high hydrogenation pressures and reaction temperatures to be effective (i.e. often requiring pressures above 197 atm and temperatures exceeding 200 °C). Selective catalysts for the reaction include copper chromite, rhenium trioxide and rhenium(VII) oxide or bimetallic catalyst.
Amines from other hydride sources
Reducing agents able to effect this reaction include metal hydrides such as lithium aluminium hydride, or lithium borohydride in mixed solvents of tetrahydrofuran and methanol.
Iron catalysis by triiron dodecacarbonyl in combination with polymethylhydrosiloxane has been reported.
Lawesson's reagent converts amides to thioamides, which then catalytically desulfurize.
Noncatalytic routes to aldehydes
Some amides can be reduced to aldehydes in the Sonn-Müller method, but most routes to aldehydes involve a well-chosen organometallic reductant.
Lithium aluminum hydride reduces an excess of N,N-disubstituted amides to an aldehyde:
- R(CO)NRR' + LiAlH4 → RCHO + HNRR'
With further reduction the alcohol is obtained.
Schwartz's reagent reduces amides to aldehydes, and so does hydrosilylation with a suitable catalyst.
References
- ^ Nishimura, Shigeo (2001). Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (1st ed.). New York: Wiley-Interscience. pp. 406–411. ISBN 9780471396987.
- March, Jerry (1985). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.). New York: Wiley. ISBN 9780471854722. OCLC 642506595.
- Mitsudome, Takato; Miyagawa, Kazuya; Maeno, Zen; Mizugaki, Tomoo; Jitsukawa, Koichiro; Yamasaki, Jun; Kitagawa, Yasutaka; Kaneda, Kiyotomi (2017-08-01). "Mild Hydrogenation of Amides to Amines over a Platinum-Vanadium Bimetallic Catalyst". Angewandte Chemie International Edition. 56 (32): 9381–9385. doi:10.1002/anie.201704199. PMID 28649715.
- Zhang, Yue; Zhang, Fan; Li, Lin; Liu, Fei; Wang, Aiqin (2022-10-07). "Highly Chemoselective Reduction of Amides to Amines over a Ruthenium‐Molybdenum Bimetallic Catalyst". ChemistrySelect. 7 (37). doi:10.1002/slct.202203030. ISSN 2365-6549. S2CID 252725710.
- Pennetier, Alex; Hernandez, Willinton Y.; Kusema, Bright T.; Streiff, Stéphane (2021-08-25). "Efficient hydrogenation of aliphatic amides to amines over vanadium-modified rhodium supported catalyst". Applied Catalysis A: General. 624: 118301. doi:10.1016/j.apcata.2021.118301. ISSN 0926-860X. S2CID 238850541.
- Cope, Arthur C.; Ciganek, Engelbert (1959). "N,N-Dimethylcyclohexylmethylamine". Organic Syntheses. 39: 19. doi:10.15227/orgsyn.039.0019.
- Wilson, C. V.; Stenberg, J. F. (1956). "Laurylmethylamine". Organic Syntheses. 36: 48. doi:10.15227/orgsyn.036.0048.
- Moffett, Robert Bruce (1953). "2,2-Dimethylpyrrolidine". Organic Syntheses. 33: 32. doi:10.15227/orgsyn.033.0032.
- Park, Chung Ho; Simmons, Howard E. (1974). "Macrocyclic Diimines: 1,10-Diazacylooctadecane". Organic Syntheses. 54: 88. doi:10.15227/orgsyn.054.0088.
- Seebach, Dieter; Kalinowski, Hans-Otto; Langer, Werner; Crass, Gerhard; Wilka, Eva-Maria (1983). "Chiral Media for Asymmetric Solvent Inductions". Organic Syntheses. 61: 24. doi:10.15227/orgsyn.061.0024.
- Ookawa, Atsuhiro; Soai, Kenso (1986). "Mixed solvents containing methanol as useful reaction media for unique chemoselective reductions within lithium borohydride". The Journal of Organic Chemistry. 51 (21): 4000–4005. doi:10.1021/jo00371a017.
- Zhou, S.; Junge, K.; Addis, D.; Das, S.; Beller, M. (2009). "A Convenient and General Iron-Catalyzed Reduction of Amides to Amines". Angewandte Chemie International Edition in English. 48 (50): 9507–9510. doi:10.1002/anie.200904677. PMID 19784999.
- Taber, Douglass F (5 June 2006). "The Boger Route to (-)-Vindoline". Org. Chem. Highlights. Discussion of (12)→(13).
- Leighty, M. W.; Spletstoser, J. T.; Georg, Gunda I. (2011). "Mild Conversion of Tertiary Amides to Aldehydes Using Cp2ZrHCl (Schwartz's Reagent)". Org. Synth. 88: 427–437. doi:10.1002/0471264229.os088.39. ISBN 978-0471264224.