| |
| Names | |
|---|---|
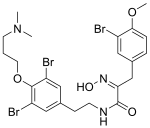
| Preferred IUPAC name (2Z)-3-(3-Bromo-4-methoxyphenyl)-N-(2-{3,5-dibromo-4-phenyl}ethyl)-2-(hydroxyimino)propanamide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C23H28Br3N3O4 |
| Molar mass | 650.198 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Aplysamine-2 is a bio-active isolate of marine sponge.
References
- Kottakota, SK; Evangelopoulos, D; Alnimr, A; Bhakta, S; McHugh, TD; Gray, M; Groundwater, PW; Marrs, EC; Perry, JD; Spilling, CD; Harburn, JJ (2012). "Synthesis and biological evaluation of purpurealidin E-derived marine sponge metabolites: aplysamine-2, aplyzanzine A, and suberedamines A and B". J Nat Prod. 75 (6): 1090–101. doi:10.1021/np300102z. PMID 22620987.
This article about an alkaloid is a stub. You can help Misplaced Pages by expanding it. |