| |
| Names | |
|---|---|
| Preferred IUPAC name 2-Benzylidene-1-benzofuran-3(2H)-one | |
| Other names
2-Benzylidenebenzofuran-3(2H)-one 2-Benzylidene-1-benzofuran-3-one | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H10O2 |
| Molar mass | 222.243 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
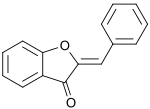
An aurone is a heterocyclic chemical compound, which is a type of flavonoid. There are two isomers of the molecule, with (E)- and (Z)-configurations. The molecule contains a benzofuran element associated with a benzylidene linked in position 2. In aurone, a chalcone-like group is closed into a 5-membered ring instead of the 6-membered ring more typical of flavonoids.
Aurone derivatives

Aurone forms the core for a family of derivatives which are known collectively as aurones. Aurones are plant flavonoids that provide yellow color to the flowers of some popular ornamental plants, such as snapdragon and cosmos. Aurones including 4'-chloro-2-hydroxyaurone (C15H11O3Cl) and 4'-chloroaurone (C15H9O2Cl) can also be found in the brown alga Spatoglossum variabile.
Most aurones are in a (Z)-configuration, which is the more stable configuration according to Austin Model 1 computation. But there are also some in the (E)-configurations such as (E)-3'-O-β-d-glucopyranosyl-4,5,6,4'-tetrahydroxy-7,2'-dimethoxyaurone, found in Gomphrena agrestis.
Biosynthesis
Aurones are biosynthesized starting from coumaryl-CoA. Aureusidin synthase catalyzes the creation of aurones from chalcones through hydroxylation and oxidative cyclization.
Applications
Some aurone derivatives possess antifungal properties and analogy with flavonoids suggests that aurones could have other biological properties.
Related compound examples
- Aureusidin
- Hispidol (6,4'-dihydroxyaurone)
- Leptosidin
- Sulfuretin (6,3',4'-trihydroxyaurone)
- 4,5,6-Trihydroxyaurone
References
- Nakayama, T (2002). "Enzymology of aurone biosynthesis". Journal of Bioscience and Bioengineering. 94 (6): 487–91. doi:10.1016/S1389-1723(02)80184-0. PMID 16233339.
- ^ Nakayama, T; Sato, T; Fukui, Y; Yonekura-Sakakibara, K; Hayashi, H; Tanaka, Y; Kusumi, T; Nishino, T (2001). "Specificity analysis and mechanism of aurone synthesis catalyzed by aureusidin synthase, a polyphenol oxidase homolog responsible for flower coloration". FEBS Letters. 499 (1–2): 107–11. doi:10.1016/S0014-5793(01)02529-7. PMID 11418122.
- ^ Atta-Ur-Rahman; Choudhary, MI; Hayat, S; Khan, AM; Ahmed, A (2001). "Two new aurones from marine brown alga Spatoglossum variabile". Chemical & Pharmaceutical Bulletin. 49 (1): 105–7. doi:10.1248/cpb.49.105. PMID 11201212.
- Ferreira, EO; Salvador, MJ; Pral, EM; Alfieri, SC; Ito, IY; Dias, DA (2004). "A new heptasubstituted (E)-aurone glucoside and other aromatic compounds of Gomphrena agrestis with biological activity" (PDF). Zeitschrift für Naturforschung C. 59 (7–8): 499–505. doi:10.1515/znc-2004-7-808. PMID 15813368. S2CID 15589214.
- Vogt, T. (2010). "Phenylpropanoid Biosynthesis". Molecular Plant. 3: 2–20. doi:10.1093/mp/ssp106. PMID 20035037.
- Sutton, Caleb L.; Taylor, Zachary E.; Farone, Mary B.; Handy, Scott T. (2017-02-15). "Antifungal activity of substituted aurones". Bioorganic & Medicinal Chemistry Letters. 27 (4): 901–903. doi:10.1016/j.bmcl.2017.01.012. PMID 28094180.
- Villemin, Didier; Martin, Benoit; Bar, Nathalie (1998). "Application of Microwave in Organic Synthesis. Dry Synthesis of 2-Arylmethylene-3(2)-naphthofuranones". Molecules. 3 (8): 88. doi:10.3390/30300088.
- Hispidol on metabolomics.jp
| Types of flavonoids | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flavonoids |
| ||||||||||||||||||||||||||||||||||||||||
| Flavonoid biosynthesis | |||||||||||||||||||||||||||||||||||||||||
| Aurones and their glycosides | |
|---|---|
| Aurones | |
| Glycosides | |