| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name Benzenecarboximidamide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.589 |
| IUPHAR/BPS | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H8N2 |
| Molar mass | 120.155 g·mol |
| Appearance | White solid |
| Density | 1.22 g/cm |
| Melting point | 64–66 °C (147–151 °F; 337–339 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
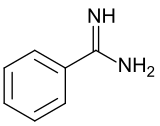
Benzamidine is an organic compound with the formula C6H5C(NH)NH2. It is the simplest aryl amidine. The compound is a white solid that is slightly soluble in water. It is usually handled as the hydrochloride salt, a white, water-soluble solid.
Structure
Benzamidine has one short C=NH bond and one longer C-NH2 bond, which are respectively 129 and 135 pm in length, respectively.
The triangular diamine group gives it a distinctive shape which shows up in difference density maps.
Applications
Benzamidine is a reversible competitive inhibitor of trypsin, trypsin-like enzymes, and serine proteases.
It is often used as a ligand in protein crystallography to prevent proteases from degrading a protein of interest. The benzamidine moiety is also found in some pharmaceuticals, such as dabigatran.
Condensation with various haloketones provides a synthetic route to 2,4-disubstituted imidazoles.
References
- Armarego, W. L. F.; Chai, Christina Li Lin (2003). Purification of Laboratory Chemicals. Amsterdam ; Boston: Butterworth-Heinemann. p. 119. ISBN 978-0-7506-7571-0. OCLC 52733960.
- ^ Li, Bryan; Chiu, Charles K-F; Hank, Richard F.; Murry, Jerry; Roth, Joshua; Tobiassen, Harry (2005). "Preparation of 2,4-Disubstituted Imidazoles: 4-(4-Methoxyphenyl)-2-Phenyl-1H-Imidazole". Organic Syntheses. 81: 105. doi:10.15227/orgsyn.081.0105.
- Barker, J.; Phillips, P. R.; Wallbridge, M. G. H.; Powell, H. R. (1996). "Benzamidine". Acta Crystallographica Section C Crystal Structure Communications. 52 (10): 2617–2619. doi:10.1107/S0108270196006282.
- Tanizawa, Kazutaka; Ishii, Shin-ichi; Hamaguchi, Kazo; Kanaoka, Yuichi (1971-05-01). "Proteolytic Enzymes. VI. Aromatic Amidines as Competitive Inhibitors of Trypsin". The Journal of Biochemistry. 69 (5): 893–899. doi:10.1093/oxfordjournals.jbchem.a129540. ISSN 0021-924X. PMID 5577153.
