| |
| Names | |
|---|---|
| Preferred IUPAC name bis(triethoxysilane) | |
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.049.888 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
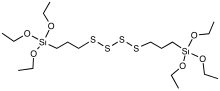
SMILES
| |
| Properties | |
| Chemical formula | C18H42O6S4Si2 |
| Molar mass | 538.95 |
| Appearance | yellow syrup |
| Density | 1.08 g/cm |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Bis(triethoxysilylpropyl)tetrasulfide is an organosulfur compound with the formula S42 (Et = C2H5). The molecule consists of two trialkoxysilyl propyl groups linked with a polysulfide. It is often sold as a mixture with the trisulfide. The compound is a colorless viscous liquid that is soluble in ordinary organic solvents such as toluene. Commercial samples often are yellowish. The compound is added to rubber compositions that contain silica filler.
Synthesis and reactivity

The compound was first prepared by the reaction of 3-(triethoxysilyl)propyl chloride with sodium tetrasulfide:
- Na2S4 + 2 ClC3H6Si(OEt)3 → S42 + 2 NaCl
Bis(triethoxysilylpropyl)tetrasulfide is a bifunctional molecule in that it contains two kinds of reactive functional groups. The tetrasulfide group is a polysulfide, which means that it consists of a chain of sulfur atoms. S-S bonds are susceptible to reduction (to thiols), attachment to metals (e.g., for protection against corrosion), and vulcanization. The triethoxysilyl groups are susceptible to hydrolysis, resulting in cross-linking via sol-gel condensation. In the usual application of this chemical, the hydrolyzed siloxy groups attach to silica particles and the polysulfide groups link to the organic polymer.
References
- Kohjiya, Shinzo; Ikeda, Yuko (2000). "Reinforcement of general-purpose grade rubbers by silica generated in situ". Rubber Chemistry and Technology. 73 (3): 534–550. doi:10.5254/1.3547604.
- Wolff, Siegfried (1996). "Chemical aspects of rubber reinforcement by fillers". Rubber Chemistry and Technology. 69 (3): 325–346. doi:10.5254/1.3538376.
- Vilmin, F.; Bottero, I.; Travert, A.; Malicki, N.; Gaboriaud, F.; Trivella, A.; Thibault-Starzyk, F. (2014). "Reactivity of Bis Tetrasulfide (TESPT) Silane Coupling Agent over Hydrated Silica: Operando IR Spectroscopy and Chemometrics Study". The Journal of Physical Chemistry C. 118 (8): 4056–4071. doi:10.1021/jp408600h.
- Thurn, Friedrich; Meyer-Simon, Eugen; Michel, Rudolf "Verfahren zur Herstellung von Organosiliziumverbindungen (Continuous manufacture of bis tetrasulfide)" Ger. Offen. (1973), DE 2212239 A1 19731004.
- Choi, S.-S.; Kim, I.-S.; Woo, C.-S. (2007). "Influence of TESPT Content on Crosslink Types and Rheological Behaviors of Natural rubber compounds reinforced with Silica". Journal of Applied Polymer Science. 106 (4): 2753–2758. doi:10.1002/app.25744.