| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 3,3-Bis(3-bromo-4-hydroxy-5-methylphenyl)-2,1λ-benzoxathiole-1,1(3H)-dione | |
| Other names
5′,5′′-Dibromo-o-cresolsulfonephthalein Bromcresol purple | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.716 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H16Br2O5S |
| Molar mass | 540.22 g·mol |
| Appearance | Purple powder |
| Melting point | 241 to 242 °C (466 to 468 °F; 514 to 515 K) (decomposition) |
| Solubility in water | < 0.1 % |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
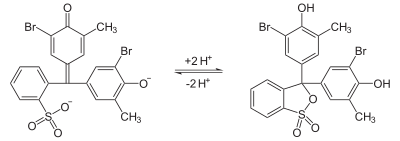
Bromocresol purple (BCP) or 5′,5″-dibromo-o-cresolsulfophthalein, is a dye of the triphenylmethane family (triarylmethane dyes) and a pH indicator. It is colored yellow below pH 5.2, and violet above pH 6.8. In its cyclic sulfonate ester form, it has a pKa value of 6.3, and is usually prepared as a 0.04% aqueous solution.
Uses

| Bromocresol purple (pH indicator) | ||
| below pH 5.2 | above pH 6.8 | |
| 5.2 | ⇌ | 6.8 |
Bromocresol purple is used in medical laboratories to measure albumin. Use of BCP in this application may provide some advantage over older methods using bromocresol green. In microbiology, it is used for staining dead cells based on their acidity, and for the isolation and assaying of lactic acid bacteria.
In photographic processing, it can be used as an additive to acid stop baths to indicate that the bath has reached neutral pH and needs to be replaced.
Bromocresol purple milk solids glucose agar is used as a medium used to distinguish dermatophytes from bacteria and other organisms in cases of ringworm fungus (T. verrucosum) infestation in cattle and other animals.
pH Indicator
Similar to bromocresol green, the structure of bromocresol purple changes with pH. Changing the level of acidity causes a shift in the equilibrium between two different structures that have different colors. In near-neutral or alkaline solution, the chemical has a sulfonate structure that gives the solution a purple color. As the pH decreases, it converts to a sultone (cyclic sulfonic ester) that colors the solution yellow. In some microbiology tests, this change is used as an indicator of bacterial growth.
See also
References
- "Bromocresol Purple". NCBI PubChem. National Center for Biotechnology Information.
- Bachmann, Lorin M.; Yu, Min; Boyd, James C.; Bruns, David E.; Miller, W. Greg (2017-03-01). "State of Harmonization of 24 Serum Albumin Measurement Procedures and Implications for Medical Decisions". Clinical Chemistry. 63 (3): 770–779. doi:10.1373/clinchem.2016.262899. ISSN 0009-9147. PMID 28073902.
- Ito, Shigenori; Yamamoto, Daisuke (2010-02-02). "Mechanism for the color change in bromocresol purple bound to human serum albumin". Clinica Chimica Acta. 411 (3): 294–295. doi:10.1016/j.cca.2009.11.019. PMID 19932090.
- Kurzweilová, H.; Sigler, K. (November 1993). "Fluorescent staining with bromocresol purple: a rapid method for determining yeast cell dead count developed as an assay of killer toxin activity". Yeast. 9 (11): 1207–1211. doi:10.1002/yea.320091107. PMID 7509098. S2CID 44782970.
- Lee, H.M.; Lee, Y. (June 2008). "A differential medium for lactic acid-producing bacteria in a mixed culture". Letters in Applied Microbiology. 46 (6): 676–681. doi:10.1111/j.1472-765X.2008.02371.x. PMID 18444977.

- Anchell, Steve (2016). The Darkroom Cookbook (4 ed.). Routledge. ISBN 9781317337607 – via Google Books.
- Kane, J.; Summerbell, R.; Sigler, L.; Krajden, S.; Land, G. (1997). Laboratory Handbook of Dermatophytes: A Clinical Guide and Laboratory Handbook of Dermatophytes and Other Filamentous Fungi from Skin, Hair, and Nails. Belmont, CA: Star Publishing Company. ISBN 9780898631579.
- Beneke, E. S.; Rogers, A. L. (1996). Medical Mycology and Human Mycoses (illustrated ed.). Belmont, CA: Star Publishing Company. pp. 85–90. ISBN 9780898631753.
- Li, Nan; Zhou, Siyu; Yang, Xingbin; Lin, Dehui (2022). "Applications of natural polysaccharide-based pH-sensitive films in food packaging: Current research and future trends". Innovative Food Science & Emerging Technologies. 82. doi:10.1016/j.ifset.2022.103200.
- "Archived copy". Archived from the original on 2022-02-16. Retrieved 2022-02-15.
{{cite web}}: CS1 maint: archived copy as title (link)