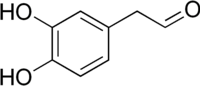
The catecholaldehyde hypothesis is a scientific theory positing that neurotoxic aldehyde metabolites of the catecholamine neurotransmitters dopamine and norepinephrine are responsible for neurodegenerative diseases involving loss of catecholaminergic neurons, for instance Parkinson's disease. The specific metabolites thought to be involved include 3,4-dihydroxyphenylacetaldehyde (DOPAL) and 3,4-dihydroxyphenylglycolaldehyde (DOPEGAL), which are formed from dopamine and norepinephrine by monoamine oxidase, respectively. These metabolites are subsequently inactivated and detoxified by aldehyde dehydrogenase (ALDH). DOPAL and DOPEGAL are monoaminergic neurotoxins in preclinical models and inhibition of and polymorphisms in ALDH are associated with Parkinson's disease. The catecholaldehyde hypothesis additionally posits that DOPAL oligomerizes with α-synuclein resulting in accumulation of oligomerized α-synuclein (i.e., synucleinopathy) and that this contributes to cytotoxicity.
See also
References
- ^ Goldstein DS (February 2020). "The catecholaldehyde hypothesis: where MAO fits in". J Neural Transm (Vienna). 127 (2): 169–177. doi:10.1007/s00702-019-02106-9. PMC 10680281. PMID 31807952.
- ^ Goldstein DS (June 2021). "The Catecholaldehyde Hypothesis for the Pathogenesis of Catecholaminergic Neurodegeneration: What We Know and What We Do Not Know". Int J Mol Sci. 22 (11): 5999. doi:10.3390/ijms22115999. PMC 8199574. PMID 34206133.
- ^ Goldstein DS, Sharabi Y (January 2019). "The heart of PD: Lewy body diseases as neurocardiologic disorders". Brain Res. 1702: 74–84. doi:10.1016/j.brainres.2017.09.033. PMC 10712237. PMID 29030055.
- Marchitti SA, Deitrich RA, Vasiliou V (June 2007). "Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase". Pharmacol Rev. 59 (2): 125–150. doi:10.1124/pr.59.2.1. PMC 2647328. PMID 17379813.
- Goldstein DS, Kopin IJ, Sharabi Y (December 2014). "Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders". Pharmacol Ther. 144 (3): 268–282. doi:10.1016/j.pharmthera.2014.06.006. PMC 4591072. PMID 24945828.
| Neurotransmitter metabolic intermediates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Catecholamines |
| ||||||||||
| Tryptophan→Serotonin |
| ||||||||||
| Serotonin→Melatonin | |||||||||||
| Trace amines | |||||||||||
| GABA | |||||||||||
This neuroscience article is a stub. You can help Misplaced Pages by expanding it. |