| |
| Names | |
|---|---|
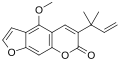
| IUPAC name 6-(2-Methylbut-3-en-2-yl)furochromen-7-one | |
| Other names Xyloltenin; 3-(α,α-dimethylallyl)psoralen | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
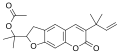
| Chemical formula | C16H14O3 |
| Molar mass | 254.285 g·mol |
| Melting point | 82–83 °C (180–181 °F; 355–356 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Chalepensin is a chemical compound of the furanocoumarin class. Originally isolated in 1967 from fringed rue (Ruta chalepensis), from which it derives its name, it has also been found in other plants of the genus Ruta including common rue (Ruta graveolens) and mountain rue (Ruta montana).
Chemical properties
Chalepensin forms colorless crystalline needles with a melting point of 82-83 °C.
Research
Chalepensin has been shown to have antifertility effects in female rats. This may be the result of toxic effects chalepensin has on the ovaries. This antifertility effect may provide some scientific evidence in support of the traditional uses of fringed rue and modern use of rue oil (oil from plants of the genus Ruta) in South America as an abortifacient.
Chalepensis has also been shown to have antibacterial activity against Streptococcus mutans and methicillin-resistant Staphylococcus aureus (MRSA).
Related compounds
Several chemical compounds that have the same core chemical structure as chalepensin are known, including chalepin, rutamarin, 5-methoxychalepensin, and 5,8-dimethoxychalepensin.
References
- Brooker, Robert M.; Eble, John N.; Starkovsky, Nicolas A. (1967). "Chalepensin, chalepin, and chalepin acetate, three novel furocoumarins from ruta chalepensis". Lloydia. 30 (1): 73–77.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kong, Y.; Lau, C.; Wat, K.; Ng, K.; But, P.; Cheng, K.; Waterman, P. (1989). "Antifertility Principle of Ruta graveolens". Planta Medica. 55 (2): 176–178. doi:10.1055/s-2006-961917. PMID 2748734.
- Touati, Driss; Atta-Ur-Rahman; Ulubelen, Ayhan (2000). "Alkaloids from Ruta montana". Phytochemistry. 53 (2): 277–279. doi:10.1016/S0031-9422(99)00486-0. PMID 10680183.
- Wu, Tian-Shung; Shi, Li-Shian; Wang, Jhi-Joung; Iou, Song-Chou; Chang, Hsien-Chang; Chen, Yuh-Pan; Kuo, Yao-Haur; Chang, Ya-Ling; Tenge, Che-Ming (2003). "Cytotoxic and Antiplatelet Aggregation Principles of Ruta Graveolens". Journal of the Chinese Chemical Society. 50: 171–178. doi:10.1002/jccs.200300024.
- ^ Ulubelen, A.; Ertugrul, L.; Birman, H.; Yigit, R.; Erseven, G.; Olgac, V. (1994). "Antifertility effects of some coumarins isolated from Ruta chalepensis andR. Chalepensis var.latifolia in rodents". Phytotherapy Research. 8 (4): 233–236. doi:10.1002/ptr.2650080409. S2CID 85292230.
- ^ Nahar, Lutfun; Al-Majmaie, Shaymaa; Al-Groshi, Afaf; Rasul, Azhar; Sarker, Satyajit D. (2021). "Chalepin and Chalepensin: Occurrence, Biosynthesis and Therapeutic Potential". Molecules. 26 (6): 1609. doi:10.3390/molecules26061609. PMC 7999183. PMID 33799365.
- Ciganda, Carmen; Laborde, Amalia (2003). "Herbal Infusions Used for Induced Abortion". Journal of Toxicology: Clinical Toxicology. 41 (3): 235–239. doi:10.1081/CLT-120021104. PMID 12807304. S2CID 44851492.
- Al-Majmaie, Shaymaa; Nahar, Lutfun; Rahman, M. Mukhlesur; Nath, Sushmita; Saha, Priyanka; Talukdar, Anupam Das; Sharples, George P.; Sarker, Satyajit D. (2021). "Anti-MRSA Constituents from Ruta chalepensis (Rutaceae) Grown in Iraq, and in Silico Studies on Two of Most Active Compounds, Chalepensin and 6-Hydroxy-rutin 3′,7-Dimethyl ether". Molecules. 26 (4): 1114. doi:10.3390/molecules26041114. PMC 7923287. PMID 33669881.