
| |
| Names | |
|---|---|
| Preferred IUPAC name (2E,4E)-1-{(3aR,8aR,13bR,16aS,17S)-17--9-methyl-2,3,8a,9,14,15-hexahydro-8H-13,16-methanobenzoindolopyrrolonaphthyridin-1(16aH)-yl}hexa-2,4-dien-1-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C32H36N4O2 |
| Molar mass | 508.666 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Communesin B is a cytotoxic chemical compound isolated from Penicillium strains found on the marine alga Ulva intestinalis. It exhibits cytotoxicity in vitro against human lung carcinoma, prostate carcinoma, colorectal carcinoma, cervical adenocarcinoma, and breast adenocarcinoma cell lines.
Biosynthesis
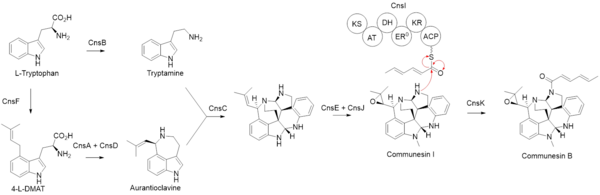
Communesin B is a dimeric indole alkaloid with a hexadienoyl moiety originating from polyketide synthesis. Biosynthesis starts with two L-tryptophan molecules processed by different pathways. The first pathway involves a decarboxylation step catalyzed by CnsB to produce tryptamine. The second pathway starts the synthesis of 4-L-dimethylallyl tryptophan by CnsF followed by further processing of CnsA and CnsD to form aurantioclavine. These two indole-containing fragments are combined through a radical oxidative coupling by CnsC, a cytochrome P450 enzyme, to form the core scaffold of communesin alkaloids. CnsE transfers a methyl group to the indole nitrogen, and CnsJ creates an epoxide ring on the dimethylallyl substituent off the ring structure to form communesin I. Separately, CnsI synthesizes a hexadienoyl group using acetyl-CoA as a starting material and extending it with two malonyl-CoA units. Then, CnsK performs N-acylation with the CnsI-synthesized hexadienoyl chain to form communesin B.
References
- Crawley SL, Funk RL (September 2003). "A synthetic approach to nomofungin/communesin B". Organic Letters. 5 (18): 3169–3171. doi:10.1021/ol034407v. PMID 12943379.
- Nicoletti R, Trincone A (February 2016). "Bioactive Compounds Produced by Strains of Penicillium and Talaromyces of Marine Origin". Marine Drugs. 14 (2): 37. doi:10.3390/md14020037. PMC 4771990. PMID 26901206.
- Pompeo, Matthew M.; Cheah, Jaime H.; Movassaghi, Mohammad (2019-09-11). "Total Synthesis and Anti-Cancer Activity of All Known Communesin Alkaloids and Related Derivatives". Journal of the American Chemical Society. 141 (36): 14411–14420. doi:10.1021/jacs.9b07397. ISSN 0002-7863. PMC 6743222. PMID 31422662.
- ^ Wei X, Wang WG, Matsuda Y (March 2022). "Branching and converging pathways in fungal natural product biosynthesis". Fungal Biology and Biotechnology. 9 (1): 6. doi:10.1186/s40694-022-00135-w. PMC 8902786. PMID 35255990.
- Pompeo MM, Cheah JH, Movassaghi M (September 2019). "Total Synthesis and Anti-Cancer Activity of All Known Communesin Alkaloids and Related Derivatives". Journal of the American Chemical Society. 141 (36): 14411–14420. doi:10.1021/jacs.9b07397. PMC 6743222. PMID 31422662.
- ^ Lin HC, Chiou G, Chooi YH, McMahon TC, Xu W, Garg NK, Tang Y (March 2015). "Elucidation of the concise biosynthetic pathway of the communesin indole alkaloids". Angewandte Chemie. 54 (10): 3004–3007. doi:10.1002/anie.201411297. PMC 4409825. PMID 25571861.