| |
| Names | |
|---|---|
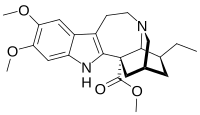
| IUPAC name Methyl (1S,15S,17S,18S)-17-ethyl-6,7-dimethoxy-3,13-diazapentacyclononadeca-2(10),4,6,8-tetraene-1-carboxylate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C23H30N2O4 |
| Molar mass | 398.503 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Conopharyngine is the major alkaloid present in the leaves and stem-bark of Tabernaemontana pachysiphon and Conopharyngia durissima. It is closely related voacangine and coronaridine. Conopharyngine pseudoindoxyl, a derivative of it, is also found in the same plant Tabernaemontana pachysiphon.
Pharmacology
It possess central nervous system stimulant activity and produces bradycardia and hypotension in cats. It has weak acetylcholinesterase inhibitory activity and significantly increases hexobarbitone induced sleeping time.
Toxicity
It has low intravenous toxicity in mice (LD50 = 143 mg/kg).
See also
References
- "Tabernaemontana pachysiphon".
- van Beek TA, de Smidt C, Verpoorte R (1985). "Phytochemical investigation of Tabernaemontana crassa". Journal of Ethnopharmacology. 14 (2–3): 315–8. doi:10.1016/0378-8741(85)90096-0. PMID 4094474.
- Renner, U.; Prins, D. A.; Stoll, W. G. (1959). "Alkaloide ausConopharyngia durissima STAPF Isovoacangin, Conopharyngin, Conodurin und Conoduramin". Helvetica Chimica Acta. 42 (5): 1572–1581. doi:10.1002/hlca.19590420519. ISSN 0018-019X.
- Crooks PA, Robinson B (October 1973). "Conopharyngine pseudoindoxyl, a new alkaloid from Tabernamontana pachysiphon Stapf. var cumminsii (Stapf.) H. Huber". The Journal of Pharmacy and Pharmacology. 25 (10): 820–3. doi:10.1111/j.2042-7158.1973.tb09948.x. PMID 4149744. S2CID 9261649.
- ^ Carroll PR, Starmer GA (May 1967). "Studies on the pharmacology of conopharyngine, an indole alkaloid of the voacanga series". British Journal of Pharmacology and Chemotherapy. 30 (1): 173–85. doi:10.1111/j.1476-5381.1967.tb02123.x. PMC 1557242. PMID 6039971.