| |
| Names | |
|---|---|
| Preferred IUPAC name Cyclopropanol | |
| Other names Cyclopropyl alcohol, Hydroxycyclopropane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.217.724 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H6O |
| Molar mass | 58.080 g·mol |
| Density | 0.917 g/mL |
| Boiling point | 101 to 102 °C (214 to 216 °F; 374 to 375 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
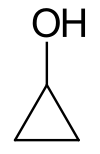
Cyclopropanol is an organic compound with the chemical formula C3H6O. It contains a cyclopropyl group with a hydroxyl group attached to it. The compound is highly unstable due to the three-membered ring, and is susceptible to reactions that open the ring. It is highly prone to rearrangement, undergoing structural isomerization to form propanal. This property is useful synthetically: cyclopropanol can be used as a synthon for the homoenolate of propanal. The chemical is also useful as a reagent to introduce a cyclopropyl group into ester, sulfate, and amine linkages. The resulting cyclopropyl-containing compounds have been used in investigations of potential antiviral drugs and of modulators of protein trafficking.
References
- Roberts, J. D.; Chambers, V. C. (1951). "Small-Ring Compounds. VI. Cyclopropanol, Cyclopropyl Bromide and Cyclopropylamine". J. Am. Chem. Soc. 73 (7): 3176–3179. doi:10.1021/ja01151a053.
- Jongejan, J. A.; Duine, J. A. (1987). "Enzymatic hydrolysis of cyclopropyl acetate. A facile method for medium- and large-scale preparations of cyclopropanol". Tetrahedron Lett. 28 (24): 2767–2768. doi:10.1016/S0040-4039(00)96204-X.
- Magrane, J. K.; Cottle, D. L. (1942). "The Reaction of Epichlorohydrin with the Grignard Reagent". J. Am. Chem. Soc. 64 (3): 484–487. doi:10.1021/ja01255a004.
- Stahl, G. W.; Cottle, D. L. (1943). "The Reaction of Epichlorohydrin with the Grignard Reagent. Some Derivatives of Cyclopropanol". J. Am. Chem. Soc. 65 (9): 1782–1783. doi:10.1021/ja01249a507.
- WO application 2009005677, Cottell, J. J.; Link, J. O. Schroeder, S. D.; Taylor, J.; Tse, W.; Vivian, R. W.; Yang, Z.-Y., "Antiviral compounds", published 2009-01-08
- WO application 2009062118, Bulawa, C. E.; Devit, M.; Elbaum, D., "Modulators of protein trafficking", published 2009-05-14