| |
| Names | |
|---|---|
| Preferred IUPAC name Dibutyl (2Z)-but-2-enedioate | |
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.027 |
| EC Number |
|
| MeSH | maleate dibutyl maleate |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H20O4 |
| Molar mass | 228.288 g·mol |
| Appearance | Colorless to yellowish liquid with a characteristic odor |
| Density | 0.99 g·cm |
| Melting point | −85 °C (−121 °F; 188 K) |
| Boiling point | 280 °C (536 °F; 553 K) |
| Solubility in water | Very hardly soluble (0.17 g·l at 20 °C) |
| Vapor pressure | 0.0027 hPa (20 °C) |
| Refractive index (nD) | 1.445 (20 °C) |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Hazard statements | H317, H373, H411 |
| Precautionary statements | P273, P280, P302+P352, P314 |
| Flash point | 141 °C (286 °F; 414 K) |
| Autoignition temperature |
265 °C (509 °F; 538 K) |
| Explosive limits |
|
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
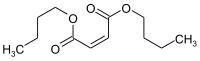
Dibutyl maleate is an organic compound with the formula (CHCO2Bu)2 (Bu = butyl). It is the diester of the unsaturated dicarboxylic acid maleic acid. It is a colorless oily liquid, although impure samples can appear yellow.
Preparation
Dibutyl maleate can be prepared by the reaction of maleic acid anhydride and 1-butanol in presence of p-toluenesulfonic acid.
Uses
Dibutyl maleate is mainly used as a plasticizer for aqueous dispersions of copolymers with vinyl acetate and as an intermediate in the preparation of other chemical compounds. With the invention of polyaspartic technology the material found another use. In this situation, an amine is reacted with a dialkyl maleate - usually diethyl maleate but also dibutyl maleate may be used- utilizing the Michael addition reaction. The resulting products, polyaspartic esters products are then used in coatings, adhesives, sealants and elastomers.
See also
References
- ^ Record of Maleinsäuredibutylester in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 3 April 2019.
- Sigma-Aldrich Co., Dibutyl maleate, 96%. Retrieved on 2019-04-03.
- R. Wen, L. Long, L. Ding, Silas Yu (2001). "Study on synthesis of dibutyl maleate". Jishou Daxue Xuebao/Journal of Jishou University. 22 (1): 78–80.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - B. Trivedi (2013). Maleic Anhydride. Springer Science & Business Media. p. 277. ISBN 978-1-4757-0940-7.
- Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for Dibutyl maletate (PDF) (Report). OECD. Retrieved 3 April 2019.
- Howarth, GA (2003-06-01). "Polyurethanes, polyurethane dispersions and polyureas: Past, present and future". Surface Coatings International Part B: Coatings Transactions. 86 (2): 111–118. doi:10.1007/BF02699621. ISSN 1476-4865. S2CID 93574741.