| |
| Names | |
|---|---|
| IUPAC name Chlorodiethylalumane | |
| Other names Chlorodiethylaluminium | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 4123259 |
| ChemSpider | |
| ECHA InfoCard | 100.002.253 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3394 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H20Al2Cl2 |
| Molar mass | 241.11 g·mol |
| Appearance | Colorless liquid |
| Density | 0.96 g/cm |
| Melting point | −74 °C (−101 °F; 199 K) |
| Boiling point | 125 to 126 °C (257 to 259 °F; 398 to 399 K) at 50 mmHg |
| Solubility in water | Reacts |
| Vapor pressure | 3 mmHg (at 60 °C) |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H225, H250, H260, H261, H314 |
| Precautionary statements | P210, P222, P223, P231+P232, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P302+P334, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P335+P334, P363, P370+P378, P402+P404, P403+P235, P405, P422, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | −18 °C (0 °F; 255 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
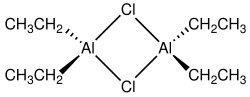
Diethylaluminium chloride, abbreviated DEAC, is an organoaluminium compound. Although often given the chemical formula (C2H5)2AlCl, it exists as a dimer, 2 It is a precursor to Ziegler-Natta catalysts employed for the production of polyolefins. The compound is also a Lewis acid, useful in organic synthesis. The compound is a colorless waxy solid, but is usually handled as a solution in hydrocarbon solvents. It is highly reactive, even pyrophoric.
Structure and bonding
Compounds of the empirical formula AlR2Cl (R = alkyl, aryl) usually exist as dimers with the formula (R2Al)2(μ-Cl)2. The bridging ligands (indicated by "μ-") are halides, not the organic substituents. The aluminium adopts a tetrahedral geometry. Each Al(III) center follows the octet rule. In contrast, triethylaluminium and trimethylaluminium feature bridging alkyl groups and these compounds violate the octet rule.
Production
Diethylaluminium chloride can be produced from ethylaluminium sesquichloride, (C2H5)3Al2Cl3, by reduction with sodium:
- 2 (C2H5)3Al2Cl3 + 3 Na → 3 (C2H5)2AlCl + Al + 3 NaCl
It is also obtained from the reaction of triethylaluminium with hydrochloric acid:
- (C2H5)3Al + HCl → (C2H5)2AlCl + C2H6
Reproportionation reactions can also be used:
- 2 (C2H5)3Al + AlCl3 → 3 (C2H5)2AlCl
- (C2H5)3Al2Cl3 + (C2H5)3Al → 3 (C2H5)2AlCl
Uses
Diethylaluminium chloride and other organoaluminium compounds are used in combination with transition metal compounds as Ziegler–Natta catalysts for the polymerization of various alkenes.
As a Lewis acid, diethylaluminium chloride also has uses in organic synthesis. For example, it is used to catalyze the Diels–Alder and ene reactions. Alternatively, it can react as a nucleophile or a proton scavenger.
Safety
Diethylaluminium chloride is not only flammable but pyrophoric.
References
- Hu, Y. J.; Jiang, H. L.; Wang, H. H., "Preparation of highly branched polyethylene with acenaphthenediimine nickel chloride/diethylaluminum chloride catalyst". Chinese Journal of Polymer Science 2006, 24 (5), 483–488.
- Yao, Y. M.; Qi, G. Z.; Shen, Q.; Hu, J. Y.; Lin, Y. H., "Reactivity and structural characterization of divalent samarium aryloxide with diethylaluminum chloride". Chinese Science Bulletin 2003, 48 (20), 2164–2167.
- ^ John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 4–40. ISBN 978-1138561632.
- ^ Snider, Barry B. (2001). "Diethylaluminum Chloride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd165. ISBN 0-471-93623-5.
- Brendhaugen, Kristen; Haaland, Arne; Novak, David P.; Østvold, Terje; Bjørseth, Alf; Powell, D. L. (1974). "The Molecular Structure of Dimethylaluminium Chloride Dimer, [(CH3)2AlCl]2 Redetermined by Gas Phase Electron Diffraction". Acta Chemica Scandinavica. 28a: 45–47. doi:10.3891/acta.chem.scand.28a-0045.
- McMahon, C. Niamh; Francis, Julie A.; Barron, Andrew R. (1997). "Molecular Atructure of 2". Journal of Chemical Crystallography. 27 (3): 191–194. doi:10.1007/BF02575988. S2CID 195242291.
- Krause, Michael J.; Orlandi, Frank; Saurage, Alfred T.; Zietz, Joseph R. (2000), "Aluminum Compounds, Organic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, pp. 592–593, doi:10.1002/14356007.a01_543, ISBN 978-3-527-30673-2
- Fisch, A. G. (2000). "Ziegler–Natta Catalysts". Kirk-Othmer Encyclopedia of Chemical Technology. Wiley. pp. 1–22. doi:10.1002/0471238961.2609050703050303.a01.pub2. ISBN 978-0-471-48494-3. S2CID 213111515.
External links
 Media related to Diethylaluminium chloride at Wikimedia Commons
Media related to Diethylaluminium chloride at Wikimedia Commons
| Aluminium compounds | |||||
|---|---|---|---|---|---|
| Al(I) |
| ||||
| Al(II) | |||||
| Al(III) |
| ||||