| |
| Names | |
|---|---|
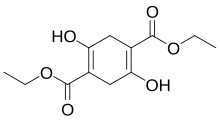
| Preferred IUPAC name Diethyl 2,5-dihydroxycyclohexa-1,4-diene-1,4-dicarboxylate | |
| Other names
2,5-Dihydroxy-1,4-cyclohexadiene-1,4-dicarboxylic acid 1,4-Bis(ethoxycarbonyl)-2,5-dihydroxy-1,4-cyclohexadiene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H16O6 |
| Molar mass | 256.254 g·mol |
| Appearance | white solid |
| Density | 1.414 g/cm |
| Melting point | 125–126 °C (257–259 °F; 398–399 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Diethylsuccinoylsuccinate is an organic compound with the formula 2 (Et = ethyl). A tetrasubstituted derivative of 1,4-cyclohexadiene, the compound is the enol tautomer of the corresponding cyclohexadione. It is produced by base-induced condensation of diethyl succinate:
- 2 EtO2CCH2CH2CO2Et 2 + 2 EtOH
Diethylsuccinylsuccinate is valued as a precursor to the quinacridone pigments. For example, it reacts with two equiv of anilines to give the diamines 2, which undergoes cyclization upon treatment with acid to give dihydroquinacridone.
When heated in the presence of acid, diethylsuccinoylsuccinate converts to 1,4-cyclohexanedione via hydrolysis of the esters followed by decarboxylation.
References
- Mez, Hans-Christian; Rihs, Gret (1973). "Chemistry of Succinylsuccinic Acid Derivatives. Part II. The crystal and molecular structure of diethyl succinylsuccinate". Helvetica Chimica Acta. 56 (8): 2766–2772. doi:10.1002/hlca.19730560812.
- ^ Nielsen, Arnold T.; Carpenter, Wayne R. (1965). "1,4-Cyclohexanedione". Organic Syntheses. 45: 25. doi:10.15227/orgsyn.045.0025.
- Hunger, K.; Herbst, W. (2012). "Pigments, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a20_371. ISBN 978-3527306732.(subscription required)
- Smith, J. Anthony; West, Richard M.; Allen, Malcolm (2004). "Acridones and Quinacridones: Novel Fluorophores for Fluorescence Lifetime Studies". Journal of Fluorescence. 14 (2): 151–171. doi:10.1023/B:JOFL.0000016287.56322.eb.
- Labana, S. S.; Labana, L. L. (1967). "Quinacridones". Chemical Reviews. 67: 1–18. doi:10.1021/cr60245a001.