| |
| Names | |
|---|---|
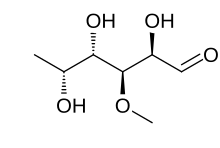
| IUPAC name 6-Deoxy-3-O-methyl-D-galactose | |
| Other names D-Digitalose; 6-Deoxy-3-O-methylgalactose; 3-Methyl-D-fucose | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H14O5 |
| Molar mass | 178.184 g·mol |
| Melting point | 106 °C (223 °F; 379 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Digitalose is a deoxy sugar that is a component of various cardiac glycosides including thevetin and emicymarin. It was first reported in 1892 as being obtained by the hydrolysis of Digtalinum verum. The chemical structure was first elucidated in 1943 by the German chemist Otto Schmidt. Chemically, it is a methyl ether of D-fucose.
See also
- Sarmentose, a related deoxy sugar
References
- ^ Digitalose, Merck Index, 12th Edition, 3202
- Kiliani (1892). "Ueber Digitalonsäure". Chem. Ber. 25 (1): 2116–2118. doi:10.1002/cber.189202501328.
- Otto Th. Schmidt; Walter Mayer; Alfred Distelmaier (1943). "Digitalose". Naturwissenschaften. 31 (21–22): 247–248. Bibcode:1943NW.....31..247S. doi:10.1007/bf01482327.