| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name disulfurous acid | |||
| Other names pyrosulfurous acid | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | H2S2O5 | ||
| Molar mass | 146.13 g·mol | ||
| Conjugate base | Disulfite | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
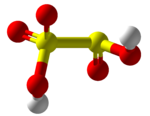
Disulfurous acid, metabisulfurous acid or pyrosulfurous acid is an oxoacid of sulfur with the formula H2S2O5. Its structure is HO−S(=O)2−S(=O)−OH. The salts of disulfurous acid are called disulfites or metabisulfites. Disulfurous acid is, like sulfurous acid (H2SO3), a phantom acid, which does not exist in the free state. In contrast to disulfate (S2O2−7), disulfite has two directly connected sulfur atoms. The oxidation state of the sulfur atom bonded to three oxygen atoms is +5 and its valence is 6, while that of the other sulfur is +3 and 4 respectively.

References
- International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 130. Electronic version.
- Holleman, Wiberg (2001). Inorganic Chemistry. Academic Press. pp. 537–540. ISBN 9780123526519.
This article about theoretical chemistry is a stub. You can help Misplaced Pages by expanding it. |
This article about an acid is a stub. You can help Misplaced Pages by expanding it. |