| This article may be too technical for most readers to understand. Please help improve it to make it understandable to non-experts, without removing the technical details. (June 2023) (Learn how and when to remove this message) |
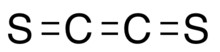
| |
| Names | |
|---|---|
| IUPAC name Ethenedithione | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C2S2 |
| Molar mass | 88.14 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Ethenedithione or ethylenedithone is an unstable chemical substance with formula S=C=C=S made from carbon and sulfur.
Ethenedithione can exist as a gas at low pressure and high temperature, but is unstable when condensed or under higher pressure.
It can be stabilized as a ligand binding two cobalt atoms.
Other occurrences as a ligand are in TpW(CO)2(C2S2) and 2Ni(C2S2)2, where Tp is trispyrazolylborate.
Formation
Ethenedithione can be made by the flash vacuum pyrolysis of 2,5-Dithiacyclopentylideneketene.
Also it has been made by dissociative ionization of tetrathiapentalenedione, and then neutralisation of ions produced.
C2S2 is made along with carbon subsulfide and carbon monosulfide, in an electric discharge in carbon disulfide vapour.
Properties
In its ground state it is a triplet state (Σg). Ethenedithione can be trapped in a matrix of solid argon without decomposition.
The infrared spectrum contains a prominent line at 1179.3 cm due to asymmetric C=S stretch of the most common isotopes.
Over 60K, ethenedithione polymerises. Possible polymerisation products include polythiene.
References
- ^ Wentrup, Curt; Kambouris, Peter; Evans, Richard A.; Owen, David; Macfarlane, Graham; Chuche, Josselin; Pommelet, Jean Claude; Cheikh, Abdelhamid Ben; Plisnier, Michel; Flammang, Robert (April 1991). "2,5-Dithiacyclopentylideneketene and ethenedithione, S:C:C:S, generated by flash vacuum pyrolysis". Journal of the American Chemical Society. 113 (8): 3130–3135. doi:10.1021/ja00008a048.
- Seidel, Wolfram W.; Meel, Matthias J.; Hughes, Stephen R.; Hupka, Florian; Villinger, Alexander (23 December 2011). "Ethenedithione (S=C=C=S): Trapping and Isomerization in a Cobalt Complex". Angewandte Chemie International Edition. 50 (52): 12617–12620. doi:10.1002/anie.201105055.
- Zhang, Zhong; Pu, Liang; Li, Qian-shu; King, R. Bruce (2014). "The facile coupling of carbon monochalcogenides to ethenedichalcogenone ligands in binuclear iron carbonyl derivatives: a theoretical study". New J. Chem. 38 (9): 4282–4289. doi:10.1039/C4NJ00740A.
- Sülzle, Detlev; Schwarz, Helmut (October 1988). "Ethylenedithione(C2S2): Generation and Characterization by Neutralization-Reionization Mass Spectrometry". Angewandte Chemie International Edition in English. 27 (10): 1337–1339. doi:10.1002/anie.198813371.
- Bohn, Robert B.; Hannachi, Yacine; Andrews, Lester (July 1992). "Production and reactions of triplet CS: matrix infrared and ultraviolet spectra of C2S2". Journal of the American Chemical Society. 114 (16): 6452–6459. doi:10.1021/ja00042a024.
- Ma, Ngai Ling; Wong, Ming Wah (1998-12-31). "Ethenedithione (S=C=C=S): Does It Obey Hund's Rule?". Angewandte Chemie International Edition. 37 (24): 3402–3404. doi:10.1002/(SICI)1521-3773(19981231)37:24<3402::AID-ANIE3402>3.0.CO;2-S. ISSN 1433-7851.
- Yranzo, Gloria I.; Elguero, José; Flammang, Robert; Wentrup, Curt (June 2001). "Formation of Cumulenes, Triple-Bonded, and Related Compounds by Flash Vacuum Thermolysis of Five-Membered Heterocycles". European Journal of Organic Chemistry. 2001 (12): 2209–2220. doi:10.1002/1099-0690(200106)2001:12<2209::AID-EJOC2209>3.0.CO;2-X.
- Zmolek, Peter B.; Sohn, Honglae; Gantzel, Peter K.; Trogler, William C. (1 February 2001). "Photopolymerization of Liquid Carbon Disulfide Produces Nanoscale Polythiene Films". Journal of the American Chemical Society. 123 (6): 1199–1207. doi:10.1021/ja003200j.