| |
| |
| Names | |
|---|---|
| Preferred IUPAC name Azidoethane | |
| Other names Ethane, azido-; 1-Azidoethane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
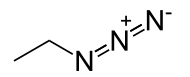
| Chemical formula | CH3CH2N3 |
| Molar mass | 71.083 g·mol |
| Appearance | liquid |
| Boiling point | 50 |
| Explosive data | |
| Shock sensitivity | High |
| Friction sensitivity | High |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH298) |
266.872 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Harmful, Explosive |
| Related compounds | |
| Related compounds | Hydrazoic acid, Chlorine azide, Methyl azide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ethyl azide (CH3CH2N3) is an explosive compound sensitive to rapid heating, shock or impact. It has exploded when heated to room temperature. When heated to decomposition it emits toxic fumes of NOx.
It is irritating to eyes, respiratory system and skin.
Uses
Ethyl azide is used for organic synthesis.
References
- Campbell, H. C.; Rice, O. K. (1935). "The Explosion of Ethyl Azide". Journal of the American Chemical Society. 57 (6): 1044–1050. doi:10.1021/ja01309a019.
- Rice, O. K.; Campbell, H. C. (1939). "The Explosion of Ethyl Azide in the Presence of Diethyl Ether". The Journal of Chemical Physics. 7 (8): 700–709. Bibcode:1939JChPh...7..700R. doi:10.1063/1.1750516.
- Rice, O. K. (1940). "The Role of Heat Conduction in Thermal Gaseous Explosions". The Journal of Chemical Physics. 8 (9): 727–733. Bibcode:1940JChPh...8..727R. doi:10.1063/1.1750808.
- Costa Cabral, B. J.; Costa, M. L.; Almoster Ferreira, M. A. (2010). "ChemInform Abstract: Molecular Structure and Ionization Energies of Azides: An ab initio Study of Hydrazoic Acid, Methyl Azide and Ethyl Azide". ChemInform. 24 (37): no. doi:10.1002/chin.199337053.