| |

| |
| Names | |
|---|---|
| Preferred IUPAC name Ethyl carbonochloridate | |
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.007.981 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1182 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | ClCO2CH2CH3 |
| Molar mass | 108.52 g·mol |
| Appearance | Colorless liquid |
| Odor | Like hydrochloric acid |
| Density | 1.1403 g/cm |
| Melting point | −81 °C (−114 °F; 192 K) |
| Boiling point | 95 °C (203 °F; 368 K) |
| Solubility in water | Decomposes |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Corrosive Flammable |
| GHS labelling: | |
| Pictograms |    
|
| Signal word | Danger |
| Hazard statements | H225, H302, H314, H330 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P260, P264, P270, P271, P280, P284, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P320, P321, P330, P363, P370+P378, P403+P233, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | 61 °C (142 °F; 334 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ethyl chloroformate is an organic compound with the chemical formula ClCO2CH2CH3. It is the ethyl ester of chloroformic acid. It is a colorless, corrosive and highly toxic liquid. It is a reagent used in organic synthesis for the introduction of the ethyl carbamate protecting group and for the formation of carboxylic anhydrides.
Preparation
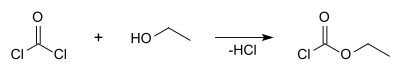
Ethyl chloroformate can be prepared using ethanol and phosgene:
Safety
Ethyl chloroformate is a highly toxic, flammable, corrosive substance. It causes severe burns when comes in contact with eyes and/or skin, can be harmful if swallowed or inhaled.
References
- Merck Index, 11th Edition, 3742.
- https://www.sigmaaldrich.com/GB/en/sds/aldrich/185892
- Protective Groups in Organic Synthesis, Third Edition, Theodora W. Greene and Peter G. M. Wuts, pages 504-506, ISBN 0-471-16019-9
- PubChem. "Ethyl chloroformate". pubchem.ncbi.nlm.nih.gov. Retrieved 2019-09-04.