| Nuclide | t1⁄2 | Yield | Q | βγ |
|---|---|---|---|---|
| (Ma) | (%) | (keV) | ||
| Tc | 0.211 | 6.1385 | 294 | β |
| Sn | 0.230 | 0.1084 | 4050 | βγ |
| Se | 0.327 | 0.0447 | 151 | β |
| Cs | 1.33 | 6.9110 | 269 | β |
| Zr | 1.53 | 5.4575 | 91 | βγ |
| Pd | 6.5 | 1.2499 | 33 | β |
| I | 16.14 | 0.8410 | 194 | βγ |
| t½ (year) |
Yield (%) |
Q (keV) |
βγ | |
|---|---|---|---|---|
| Eu | 4.76 | 0.0803 | 252 | βγ |
| Kr | 10.76 | 0.2180 | 687 | βγ |
| Cd | 14.1 | 0.0008 | 316 | β |
| Sr | 28.9 | 4.505 | 2826 | β |
| Cs | 30.23 | 6.337 | 1176 | βγ |
| Sn | 43.9 | 0.00005 | 390 | βγ |
| Sm | 94.6 | 0.5314 | 77 | β |
Nuclear fission splits a heavy nucleus such as uranium or plutonium into two lighter nuclei, which are called fission products. Yield refers to the fraction of a fission product produced per fission.
Yield can be broken down by:
- Individual isotope
- Chemical element spanning several isotopes of different mass number but same atomic number.
- Nuclei of a given mass number regardless of atomic number. Known as "chain yield" because it represents a decay chain of beta decay.
Isotope and element yields will change as the fission products undergo beta decay, while chain yields do not change after completion of neutron emission by a few neutron-rich initial fission products (delayed neutrons), with half-life measured in seconds.
A few isotopes can be produced directly by fission, but not by beta decay because the would-be precursor with atomic number one less is stable and does not decay (atomic number grows by 1 during beta decay). Chain yields do not account for these "shadowed" isotopes; however, they have very low yields (less than a millionth as much as common fission products) because they are far less neutron-rich than the original heavy nuclei.
Yield is usually stated as percentage per fission, so that the total yield percentages sum to 200%. Less often, it is stated as percentage of all fission products, so that the percentages sum to 100%. Ternary fission, about 0.2–0.4% of fissions, also produces a third light nucleus such as helium-4 (90%) or tritium (7%).
Mass vs. yield curve

If a graph of the mass or mole yield of fission products against the atomic number of the fragments is drawn then it has two peaks, one in the area zirconium through to palladium and one at xenon through to neodymium. This is because the fission event causes the nucleus to split in an asymmetric manner, as nuclei closer to magic numbers are more stable.
Yield vs. Z - This is a typical distribution for the fission of uranium. Note that in the calculations used to make this graph the activation of fission products was ignored and the fission was assumed to occur in a single moment rather than a length of time. In this bar chart results are shown for different cooling times (time after fission).
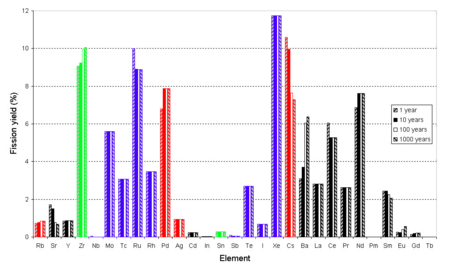
Because of the stability of nuclei with even numbers of protons and/or neutrons the curve of yield against element is not a smooth curve. It tends to alternate.
In general, the higher the energy of the state that undergoes nuclear fission, the more likely a symmetric fission is, hence as the neutron energy increases and/or the energy of the fissile atom increases, the valley between the two peaks becomes more shallow; for instance, the curve of yield against mass for Pu-239 has a more shallow valley than that observed for U-235, when the neutrons are thermal neutrons. The curves for the fission of the later actinides tend to make even more shallow valleys. In extreme cases such as Fm, only one peak is seen.
Yield is usually expressed relative to number of fissioning nuclei, not the number of fission product nuclei, that is, yields should sum to 200%.
The table in the next section ("Ordered by yield") gives yields for notable radioactive (with half-lives greater than one year, plus iodine-131) fission products, and (the few most absorptive) neutron poison fission products, from thermal neutron fission of U-235 (typical of nuclear power reactors), computed from .
The yields in the table sum to only 45.5522%, including 34.8401% which have half-lives greater than one year:
| t½ in years | Yield |
|---|---|
| 1 to 5 | 2.7252% |
| 10 to 100 | 12.5340% |
| 2 to 300,000 | 6.1251% |
| 1.5 to 16 million | 13.4494% |
The remainder and the unlisted 54.4478% decay with half-lives less than one year into nonradioactive nuclei.
This is before accounting for the effects of any subsequent neutron capture; e.g.:
- Xe capturing a neutron and becoming nearly stable Xe, rather than decaying to Cs which is radioactive with a half-life of 2.3 million years
- Nonradioactive Cs capturing a neutron and becoming Cs, which is radioactive with a half-life of 2 years
- Many of the fission products with mass 147 or greater such as Pm, Sm, Sm, and Eu have significant cross sections for neutron capture, so that one heavy fission product atom can undergo multiple successive neutron captures.
Besides fission products, the other types of radioactive products are
- plutonium containing Pu, Pu, Pu, Pu, and Pu,
- minor actinides including Np, Am, Am, curium isotopes, and perhaps californium
- reprocessed uranium containing U and other isotopes
- tritium
- activation products of neutron capture by the reactor or bomb structure or the environment
Fission products from U-235
| Yield | Element | Isotope | Halflife | Comment |
|---|---|---|---|---|
| 6.7896% | Caesium | Cs → Cs | 2.065 y | Neutron capture (29 barns) slowly converts stable Cs to Cs, which itself is low-yield because beta decay stops at Xe; can be further converted (140 barns) to Cs. |
| 6.3333% | Iodine, xenon | I → Xe | 6.57 h | Most important neutron poison; neutron capture converts 10–50% of Xe to Xe; remainder decays (9.14h) to Cs (2.3 My). |
| 6.2956% | Zirconium | Zr | 1.53 My | Long-lived fission product also produced by neutron activation in zircalloy cladding. |
| 6.1% | Molybdenum | Mo | 65.94 h | Its daughter nuclide Tc is important in medical diagnosing. |
| 6.0899% | Caesium | Cs | 30.17 y | Source of most of the decay heat from years to decades after irradiation, together with Sr. |
| 6.0507% | Technetium | Tc | 211 ky | Candidate for disposal by nuclear transmutation. |
| 5.7518% | Strontium | Sr | 28.9 y | Source of much of the decay heat together with Cs on the timespan of years to decades after irradiation. Formerly used in radioisotope thermoelectric generators. |
| 2.8336% | Iodine | I | 8.02 d | Reason for the use of potassium iodide tablets after nuclear accidents or nuclear bomb explosions. |
| 2.2713% | Promethium | Pm | 2.62 y | beta decays to very long lived Samarium-147 (half life>age of the universe); has seen some use in radioisotope thermoelectric generators |
| 1.0888% | Samarium | Sm | Observationally stable | 2nd most significant neutron poison. |
| 0.9% | Iodine | I | 15.7 My | Long-lived fission product. Candidate for disposal by nuclear transmutation. |
| 0.4203% | Samarium | Sm | 90 y | Neutron poison; most will be converted to stable Sm. |
| 0.3912% | Ruthenium | Ru | 373.6 d | ruthenium tetroxide is volatile and chemically aggressive; daughter nuclide Rh decays quickly to stable Pd |
| 0.2717% | Krypton | Kr | 10.78 y | noble gas; has some uses in industry to detect fine cracks in materials via autoradiography |
| 0.1629% | Palladium | Pd | 6.5 My | Long-lived fission product; hampers extraction of stable isotopes of platinum group metals for use due to chemical similarity. |
| 0.0508% | Selenium | Se | 327 ky | |
| 0.0330% | Europium, gadolinium | Eu → Gd | 4.76 y | Both neutron poisons, most will be destroyed while fuel still in use. |
| 0.0297% | Antimony | Sb | 2.76 y | |
| 0.0236% | Tin | Sn | 230 ky | |
| 0.0065% | Gadolinium | Gd | stable | Neutron poison. |
| 0.0003% | Cadmium | Cd | 14.1 y | Neutron poison, most will be destroyed while fuel still in use. |

Cumulative fission yields
Cumulative fission yields give the amounts of nuclides produced either directly in the fission or by decay of other nuclides.
| Product | Thermal fission yield | Fast fission yield | 14-MeV fission yield |
|---|---|---|---|
1H |
0.00171 ± 0.00018 | 0.00269 ± 0.00044 | 0.00264 ± 0.00045 |
1H |
0.00084 ± 0.00015 | 0.00082 ± 0.00012 | 0.00081 ± 0.00012 |
1H |
0.0108 ± 0.0004 | 0.0108 ± 0.0004 | 0.0174 ± 0.0036 |
2He |
0.0108 ± 0.0004 | 0.0108 ± 0.0004 | 0.0174 ± 0.0036 |
2He |
0.1702 ± 0.0049 | 0.17 ± 0.0049 | 0.1667 ± 0.0088 |
35Br |
1.304 ± 0.012 | 1.309 ± 0.043 | 1.64 ± 0.31 |
36Kr |
0.000285 ± 0.000076 | 0.00044 ± 0.00016 | 0.038 ± 0.012 |
36Kr |
0.286 ± 0.021 | 0.286 ± 0.026 | 0.47 ± 0.1 |
36Kr |
1.303 ± 0.012 | 1.307 ± 0.043 | 1.65 ± 0.31 |
38Sr |
5.73 ± 0.13 | 5.22 ± 0.18 | 4.41 ± 0.18 |
40Zr |
6.502 ± 0.072 | 6.349 ± 0.083 | 5.07 ± 0.19 |
41Nb |
0.00000042 ± 0.00000011 | 2.90±0.770 × 10 | 0.00004 ± 0.000015 |
41Nb |
6.498 ± 0.072 | 6.345 ± 0.083 | 5.07 ± 0.19 |
41Nb |
0.0702 ± 0.0067 | 0.0686 ± 0.0071 | 0.0548 ± 0.0072 |
42Mo |
0 ± 0 | 0 ± 0 | 0 ± 0 |
42Mo |
8.70 × 10 ± 3.20 × 10 | 0 ± 0 | 6.20 × 10 ± 2.50 × 10 |
42Mo |
0.00042 ± 0.00015 | 0.000069 ± 0.000025 | 0.0033 ± 0.0015 |
42Mo |
6.132 ± 0.092 | 5.8 ± 0.13 | 5.02 ± 0.13 |
43Tc |
6.132 ± 0.092 | 5.8 ± 0.13 | 5.02 ± 0.13 |
44Ru |
3.103 ± 0.084 | 3.248 ± 0.042 | 3.14 ± 0.11 |
44Ru |
0.41 ± 0.011 | 0.469 ± 0.036 | 2.15 ± 0.59 |
45Rh |
0.41 ± 0.011 | 0.469 ± 0.036 | 2.15 ± 0.59 |
50Sn |
0.00106 ± 0.00011 | 0.0039 ± 0.00091 | 0.142 ± 0.023 |
51Sb |
0.000000366 ± 0.000000098 | 0.0000004 ± 0.00000014 | 0.00193 ± 0.00068 |
51Sb |
0.000089 ± 0.000021 | 0.000112 ± 0.000034 | 0.027 ± 0.01 |
51Sb |
0.026 ± 0.0014 | 0.067 ± 0.011 | 1.42 ± 0.42 |
52Te |
4.276 ± 0.043 | 4.639 ± 0.065 | 3.85 ± 0.16 |
53I |
0.706 ± 0.032 | 1.03 ± 0.26 | 1.59 ± 0.18 |
53I |
2.878 ± 0.032 | 3.365 ± 0.054 | 4.11 ± 0.14 |
53I |
6.59 ± 0.11 | 6.61 ± 0.13 | 5.42 ± 0.4 |
53I |
6.39 ± 0.22 | 6.01 ± 0.18 | 4.8 ± 1.4 |
54Xe |
0 ± 0 | 0 ± 0 | 0.00108 ± 0.00048 |
54Xe |
0.000038 ± 0.0000098 | 0.000152 ± 0.000055 | 0.038 ± 0.014 |
54Xe |
0.0313 ± 0.003 | 0.0365 ± 0.0031 | 0.047 ± 0.0049 |
54Xe |
6.6 ± 0.11 | 6.61 ± 0.13 | 5.57 ± 0.41 |
54Xe |
0.189 ± 0.015 | 0.19 ± 0.015 | 0.281 ± 0.049 |
54Xe |
6.61 ± 0.22 | 6.32 ± 0.18 | 6.4 ± 1.8 |
54Xe |
1.22 ± 0.12 | 1.23 ± 0.13 | 2.17 ± 0.66 |
55Cs |
0.0000121 ± 0.0000032 | 0.0000279 ± 0.0000073 | 0.0132 ± 0.0035 |
55Cs |
6.221 ± 0.069 | 5.889 ± 0.096 | 5.6 ± 1.3 |
56Ba |
6.314 ± 0.095 | 5.959 ± 0.048 | 4.474 ± 0.081 |
57La |
6.315 ± 0.095 | 5.96 ± 0.048 | 4.508 ± 0.081 |
58Ce |
5.86 ± 0.15 | 5.795 ± 0.081 | 4.44 ± 0.2 |
58Ce |
5.474 ± 0.055 | 5.094 ± 0.076 | 3.154 ± 0.038 |
59Pr |
5.474 ± 0.055 | 5.094 ± 0.076 | 3.155 ± 0.038 |
60Nd |
6.30 × 10 ± 1.70 × 10 | 1.70 × 10 ± 4.80 × 10 | 0.0000137 ± 0.0000049 |
60Nd |
5.475 ± 0.055 | 5.094 ± 0.076 | 3.155 ± 0.038 |
60Nd |
2.232 ± 0.04 | 2.148 ± 0.028 | 1.657 ± 0.045 |
61Pm |
2.232 ± 0.04 | 2.148 ± 0.028 | 1.657 ± 0.045 |
61Pm |
5.00 × 10 ± 1.70 × 10 | 7.40 × 10 ± 2.50 × 10 | 0.0000013 ± 0.00000042 |
61Pm |
0.000000104 ± 0.000000039 | 1.78 × 10 ± 6.60 × 10 | 0.0000048 ± 0.0000018 |
61Pm |
1.053 ± 0.021 | 1.064 ± 0.03 | 0.557 ± 0.09 |
61Pm |
0.4204 ± 0.0071 | 0.431 ± 0.015 | 0.388 ± 0.061 |
62Sm |
0.000000149 ± 0.000000041 | 2.43 × 10 ± 6.80 × 10 | 0.0000058 ± 0.0000018 |
62Sm |
0.000061 ± 0.000022 | 0.0000201 ± 0.0000077 | 0.00045 ± 0.00018 |
62Sm |
0.4204 ± 0.0071 | 0.431 ± 0.015 | 0.388 ± 0.061 |
62Sm |
0.1477 ± 0.0071 | 0.1512 ± 0.0097 | 0.23 ± 0.015 |
63Eu |
0.4204 ± 0.0071 | 0.431 ± 0.015 | 0.388 ± 0.061 |
63Eu |
3.24 × 10 ± 8.50 × 10 | 0 ± 0 | 3.30 × 10 ± 1.10 × 10 |
63Eu |
0.000000195 ± 0.000000064 | 4.00 × 10 ± 1.10 × 10 | 0.0000033 ± 0.0000011 |
63Eu |
0.0308 ± 0.0013 | 0.044 ± 0.01 | 0.088 ± 0.014 |
| Product | Thermal fission yield | Fast fission yield | 14-MeV fission yield |
|---|---|---|---|
1H |
0.00408 ± 0.00041 | 0.00346 ± 0.00057 | - |
1H |
0.00135 ± 0.00019 | 0.00106 ± 0.00016 | - |
1H |
0.0142 ± 0.0007 | 0.0142 ± 0.0007 | - |
2He |
0.0142 ± 0.0007 | 0.0142 ± 0.0007 | - |
2He |
0.2192 ± 0.009 | 0.219 ± 0.009 | - |
35Br |
0.574 ± 0.026 | 0.617 ± 0.049 | - |
36Kr |
0.00175 ± 0.0006 | 0.00055 ± 0.0002 | - |
36Kr |
0.136 ± 0.014 | 0.138 ± 0.017 | - |
36Kr |
0.576 ± 0.026 | 0.617 ± 0.049 | - |
38Sr |
2.013 ± 0.054 | 2.031 ± 0.057 | - |
40Zr |
4.949 ± 0.099 | 4.682 ± 0.098 | - |
41Nb |
0.0000168 ± 0.0000045 | 0.00000255 ± 0.00000069 | - |
41Nb |
4.946 ± 0.099 | 4.68 ± 0.098 | - |
41Nb |
0.0535 ± 0.0066 | 0.0506 ± 0.0062 | - |
42Mo |
0 ± 0 | 0 ± 0 | - |
42Mo |
3.60 × 10 ± 1.30 × 10 | 4.80 × 10 ± 1.70 × 10 | - |
42Mo |
0.0051 ± 0.0018 | 0.0017 ± 0.00062 | - |
42Mo |
6.185 ± 0.056 | 5.82 ± 0.13 | - |
43Tc |
6.184 ± 0.056 | 5.82 ± 0.13 | - |
44Ru |
6.948 ± 0.083 | 6.59 ± 0.16 | - |
44Ru |
4.188 ± 0.092 | 4.13 ± 0.24 | - |
45Rh |
4.188 ± 0.092 | 4.13 ± 0.24 | - |
50Sn |
0.0052 ± 0.0011 | 0.0053 ± 0.0012 | - |
51Sb |
0.000024 ± 0.0000063 | 0.0000153 ± 0.000005 | - |
51Sb |
0.00228 ± 0.00049 | 0.00154 ± 0.00043 | - |
51Sb |
0.117 ± 0.015 | 0.138 ± 0.022 | - |
52Te |
5.095 ± 0.094 | 4.92 ± 0.32 | - |
53I |
1.407 ± 0.086 | 1.31 ± 0.13 | - |
53I |
3.724 ± 0.078 | 4.09 ± 0.12 | - |
53I |
6.97 ± 0.13 | 6.99 ± 0.33 | - |
53I |
6.33 ± 0.23 | 6.24 ± 0.22 | - |
54Xe |
0.00000234 ± 0.00000085 | 0.0000025 ± 0.0000012 | - |
54Xe |
0.00166 ± 0.00056 | 0.00231 ± 0.00085 | - |
54Xe |
0.0405 ± 0.004 | 0.0444 ± 0.0044 | - |
54Xe |
6.99 ± 0.13 | 7.03 ± 0.33 | - |
54Xe |
0.216 ± 0.016 | 0.223 ± 0.021 | - |
54Xe |
7.36 ± 0.24 | 7.5 ± 0.23 | - |
54Xe |
1.78 ± 0.21 | 1.97 ± 0.25 | - |
55Cs |
0.00067 ± 0.00018 | 0.00115 ± 0.0003 | - |
55Cs |
6.588 ± 0.08 | 6.35 ± 0.12 | - |
56Ba |
5.322 ± 0.059 | 5.303 ± 0.074 | - |
57La |
5.333 ± 0.059 | 5.324 ± 0.075 | - |
58Ce |
5.205 ± 0.073 | 5.01 ± 0.16 | - |
58Ce |
3.755 ± 0.03 | 3.504 ± 0.053 | - |
59Pr |
3.756 ± 0.03 | 3.505 ± 0.053 | - |
60Nd |
0.00000145 ± 0.0000004 | 0.00000251 ± 0.00000072 | - |
60Nd |
3.756 ± 0.03 | 3.505 ± 0.053 | - |
60Nd |
2.044 ± 0.039 | 1.929 ± 0.046 | - |
61Pm |
2.044 ± 0.039 | 1.929 ± 0.046 | - |
61Pm |
0.0000056 ± 0.0000019 | 0.000012 ± 0.000004 | - |
61Pm |
0.0000118 ± 0.0000044 | 0.000029 ± 0.000011 | - |
61Pm |
1.263 ± 0.032 | 1.275 ± 0.056 | - |
61Pm |
0.776 ± 0.018 | 0.796 ± 0.037 | - |
62Sm |
0.0000168 ± 0.0000046 | 0.000039 ± 0.000011 | - |
62Sm |
0.00227 ± 0.00078 | 0.0051 ± 0.0019 | - |
62Sm |
0.776 ± 0.018 | 0.797 ± 0.037 | - |
62Sm |
0.38 ± 0.03 | 0.4 ± 0.18 | - |
63Eu |
0.776 ± 0.018 | 0.797 ± 0.037 | - |
63Eu |
0.000000195 ± 0.00000005 | 0.00000048 ± 0.00000014 | - |
63Eu |
0.000049 ± 0.000012 | 0.000127 ± 0.000043 | - |
63Eu |
0.174 ± 0.03 | 0.171 ± 0.054 | - |
| JEFF-3.1 |
Joint Evaluated Fission and Fusion File, Incident-neutron data, http://www-nds.iaea.org/exfor/endf00.htm, 2 October 2006; see also A. Koning, R. Forrest, M. Kellett, R. Mills, H. Henriksson, Y. Rugama, The JEFF-3.1 Nuclear Data Library, JEFF Report 21, OECD/NEA, Paris, France, 2006, ISBN 92-64-02314-3. |

Ordered by mass number
Decays, even if lengthy, are given down to the stable nuclide.
Decays with half lives longer than a century are marked with a single asterisk (*), while decays with a half life longer than a hundred million years are marked with two asterisks (**).
| Yield | Isotope | |||
|---|---|---|---|---|
| 0.0508% | selenium-79→* | bromine-79 | ||
| 0.2717% | krypton-85→ | rubidium-85 | ||
| 5.7518% | strontium-90 → | yttrium-90→ | zirconium-90 | |
| 6.2956% | zirconium-93 →* | niobium-93 | ||
| 6.0507% | technetium-99→* | ruthenium-99 | ||
| 0.3912% | ruthenium-106→ | rhodium-106→ | palladium-106 | |
| 0.1629% | palladium-107→* | silver-107 | ||
| 0.0003% | cadmium-113m→ | cadmium-113 (essentially stable)→** | indium-113 | |
| 0.0297% | antimony-125→ | tellurium-125m→ | tellurium-125 | |
| 0.0236% | tin-126 →* | antimony-126→ | tellurium-126 | |
| 0.9% | iodine-129→* | xenon-129 | ||
| 2.8336% | iodine-131→ | xenon-131 | ||
| 6.7896% | caesium-133 → | caesium-134→ | barium-134 | |
| 6.3333% | iodine-135 → | xenon-135 → | caesium-135→* | barium-135 |
| 6.3333% | iodine-135 → | xenon-135 → | xenon-136 (essentially stable)→** | barium-136 |
| 6.0899% | caesium-137→ | barium-137 | ||
| 2.2713% | promethium-147→ | samarium-147→* | neodymium-143 | |
| 1.0888% | samarium-149 | |||
| 0.4203% | samarium-151 | |||
| 0.0330% | europium-155 → | gadolinium-155 | ||
| 0.0065% | gadolinium-157 |
Half lives, decay modes, and branching fractions
| Nuclide | Half-life | Decay mode | Branching fraction | Source | Notes |
|---|---|---|---|---|---|
35Br |
2.9 ± 0.06 m | β | 1.0 | ||
36Kr |
10.752 ± 0.023 y | β | 1.0 | ||
36Kr |
4.48 ± 0.008 h | IT | 0.214 ± 0.005 | ||
| β | 0.786 ± 0.005 | ||||
38Sr |
28.8 ± 0.07 y | β | 1.0 | ||
40Zr |
64.032 ± 0.006 d | β | 1.0 | ||
41Nb |
(7.3 ± 0.9) × 10 d | β | 1.0 | ||
41Nb |
3.61 ± 0.03 d | β | 0.025 ± 0.001 | ||
| IT | 0.975 ± 0.001 | ||||
41Nb |
34.985 ± 0.012 d | β | 1.0 | ||
43Tc |
(2.111 ± 0.012) × 10 y | β | 1.0 | ||
44Ru |
39.247 ± 0.013 d | β | 1.0 | ||
44Ru |
1.018 ± 0.005 y | β | 1.0 | ||
45Rh |
30.1 ± 0.3 s | β | 1.0 | ||
50Sn |
55 ± 5 y | β | 0.224 ± 0.02 | ||
| IT | 0.776 ± 0.02 | ||||
51Sb |
2.7238 ± 0.0002 d | EC | 0.0241 ± 0.0012 | ||
| β | 0.9759 ± 0.0012 | ||||
51Sb |
60.2 ± 0.03 d | β | 1.0 | ||
51Sb |
2.7584 ± 0.0006 y | β | 1.0 | ||
53I |
(5.89 ± 0.23) × 10 d | β | 1.0 | ||
53I |
8.0233 ± 0.0019 d | β | 1.0 | ||
53I |
20.87 ± 0.08 h | β | 1.0 | ||
53I |
6.57 ± 0.02 h | β | 1.0 | ||
54Xe |
11.930 ± 0.016 d | IT | 1.0 | ||
54Xe |
5.243 ± 0.001 d | β | 1.0 | ||
54Xe |
2.19 ± 0.01 d | IT | 1.0 | ||
54Xe |
9.14 ± 0.02 h | β | 1.0 | ||
54Xe |
15.29 ± 0.05 m | β | 0.003 ± 0.003 | ||
| IT | 0.997 ± 0.003 | ||||
55Cs |
2.063 ± 0.003 y | EC | 0.000003 ± 0.000001 | ||
| β | 0.999997 ± 0.000001 | ||||
55Cs |
30.05 ± 0.08 y | β | 1.0 | ||
56Ba |
12.753 ± 0.004 d | β | 1.0 | ||
57La |
1.67850 ± 0.00017 d | β | 1.0 | ||
58Ce |
32.508 ± 0.010 d | β | 1.0 | ||
58Ce |
285.1 ± 0.6 d | β | 1.0 | ||
59Pr |
17.28 ± 0.05 m | β | 1.0 | ||
60Nd |
10.98 ± 0.01 d | β | 1.0 | ||
61Pm |
2.6234 ± 0.0002 y | β | 1.0 | ||
61Pm |
41.29 ± 0.11 d | IT | 0.042 ± 0.007 | ||
| β | 0.958 ± 0.007 | ||||
61Pm |
5.368 ± 0.002 d | β | 1.0 | ||
61Pm |
2.2117 ± 0.0021 d | β | 1.0 | ||
61Pm |
1.1833 ± 0.0017 d | β | 1.0 | ||
62Sm |
90 ± 6 y | β | 1.0 | ||
62Sm |
1.938 ± 0.010 d | β | 1.0 | ||
63Eu |
(4.941 ± 0.007) × 10 d | β | 0.279 ± 0.003 | ||
| EC | 0.721 ± 0.003 | ||||
63Eu |
(3.1381 ± 0.0014) × 10 d | EC | 0.00018 ± 0.00013 | ||
| β | 0.99982 ± 0.00013 | ||||
63Eu |
4.753 ± 0.016 y | β | 1.0 |
- β decay branches of 0.9982 ± 0.0002 to Kr-85m and 0.0018 ± 0.0002 to Kr-85.
- ENSDF branching fractions: 0.944 ± 0.007 for IT and 0.056 ± 0.007 for β.
- β decay branch of 0.0288 ± 0.0002 to Xe-133m.
- Branching fractions were averaged from ENSDF database.
- Branching fractions were adopted from ENSDF database.
- ^ Branching fractions were adopted from LNHB data.
Ordered by thermal neutron absorption cross section
| Barns | Yield | Isotope | t½ | Comment |
|---|---|---|---|---|
| 2,650,000 | 6.3333% | I → Xe | 6.57 h | Most important neutron poison; neutron capture rapidly converts Xe to Xe; remainder decays (9.14 h) to Cs (2.3 My). |
| 254,000 | 0.0065% | Gd | ∞ | Neutron poison, but low yield. |
| 40,140 | 1.0888% | Sm | ∞ | 2nd most important neutron poison. |
| 20,600 | 0.0003% | Cd | 14.1 y | Most will be destroyed by neutron capture. |
| 15,200 | 0.4203% | Sm | 90 y | Most will be destroyed by neutron capture. |
| 3,950 60,900 |
0.0330% | Eu → Gd | 4.76 y | Both neutron poisons. |
| 96 | 2.2713% | Pm | 2.62 y | Suitable for radioisotope thermoelectric generators with annual or semi-annual refueling. |
| 80 | 2.8336% | I | 8.02 d | |
| 29 140 |
6.7896% | Cs → Cs | ∞ 2.065 y |
Neutron capture converts a few percent of nonradioactive Cs to Cs, which has very low direct yield because beta decay stops at Xe; further capture will add to long-lived Cs. |
| 20 | 6.0507% | Tc | 211 ky | Candidate for disposal by nuclear transmutation. |
| 18 | 0.6576% | I | 15.7 My | Candidate for disposal by nuclear transmutation. |
| 2.7 | 6.2956% | Zr | 1.53 My | Transmutation impractical. |
| 1.8 | 0.1629% | Pd | 6.5 My | |
| 1.66 | 0.2717% | Kr | 10.78 y | |
| 0.90 | 5.7518% | Sr | 28.9 y | |
| 0.15 | 0.3912% | Ru | 373.6 d | |
| 0.11 | 6.0899% | Cs | 30.17 y | |
| 0.0297% | Sb | 2.76 y | ||
| 0.0236% | Sn | 230 ky | ||
| 0.0508% | Se | 327 ky |
References
- "fissionyield". Archived from the original on 2007-05-28. Retrieved 2007-06-10.
- Möller, P; Madland, DG; Sierk, AJ; Iwamoto, A (15 February 2001). "Nuclear fission modes and fragment mass asymmetries in a five-dimensional deformation space". Nature. 409 (6822): 785–790. Bibcode:2001Natur.409..785M. doi:10.1038/35057204. PMID 11236985. S2CID 9754793.
- Purkayastha, B. C., and G. R. Martin. "The yields of 129I in natural and in neutron-induced fission of uranium." Canadian Journal of Chemistry 34.3 (1956): 293-300.
- ^ "Cumulative Fission Yields". www-nds.iaea.org. IAEA. Retrieved 11 November 2016.
- "Half-lives and decay branching fractions for activation products". www-nds.iaea.org. IAEA. Retrieved 11 November 2016.
- ^ Evaluated Nuclear Structure Data File, http://www-nds.iaea.org/ensdf/, 26 January 2006.
- ^ M.-M. Bé, V. Chisté, C. Dulieu, E. Browne, V. Chechev, N. Kuzmenko, R. Helmer, A. Nichols, E. Schönfeld, R. Dersch, Monographie BIPM-5, Table of Radionuclides, Vol. 2 - A = 151 to 242, 2004.
- ^ Laboratoire National Henri Becquerel, Recommended Data, http://www.nucleide.org/DDEP_WG/DDEPdata.htm Archived 2021-02-13 at the Wayback Machine, 16 January 2006.
- ^ M.-M. Bé, V.P. Chechev, R. Dersch, O.A.M. Helene, R.G. Helmer, M. Herman, S. Hlavác, A. Marcinkowski, G.L. Molnár, A.L. Nichols, E. Schönfeld, V.R. Vanin, M.J. Woods, IAEA CRP "Update of X-ray and Gamma-ray Decay Data Standards for Detector Calibration and Other Applications", IAEA Scientific and Technical Information report STI/PUB/1287, May 2007, International Atomic Energy Agency, Vienna, Austria, ISBN 92-0-113606-4.
External links
- HANDBOOK OF NUCLEAR DATA FOR SAFEGUARDS: DATABASE EXTENSIONS, AUGUST 2008
 The Live Chart of Nuclides - IAEA Color-map of yields, and detailed data by click on a nuclide.
The Live Chart of Nuclides - IAEA Color-map of yields, and detailed data by click on a nuclide.