| |
| Names | |
|---|---|
| Other names Sulfurofluoridoite | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | FO2S |
| Molar mass | 83.06 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
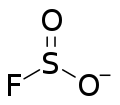
Fluorosulfite is an ion with the formula SO2F. The term is also used for compounds or salts containing this group. Fluorosulfite was discovered in 1953 by F Seel and H Meier.
Organic compounds with the name "fluorosulfite" contain the group -OS(O)F.
Preparation
can be prepared from OSF4 and Me3SiNMe2. Alkali metal fluorosulfites can be made by soaking the metal fluoride in liquid sulfur dioxide for a few days. β-CsSO2F converts to α-CsSO2F when heated to 110 °C for a couple of days but remains stable below 50 °C.
Properties
The fluorosulfite ion is tetrahedral, with sulfur at the top. The oxygen to sulfur bonds are 147.8 pm and the fluorine to sulfur bond is >169.0 pm long. In solid ionic fluorosulfites, the ion is not fixed in orientation and continuously turns around resulting in dynamic disorder. At room temperature this turning rate is from 2×10 to 10 Hz. When cooled the rate of rotation slows, and can be frozen in place, resulting in static disorder.
Fluorosulfite is isoelectronic with chloryl fluoride (ClO2F) and in compounds it resembles chlorate (ClO3).
The heat of formation from fluoride (F) and sulfur dioxide (SO2) is 50 kcal mol.
Reactions
Fluorosulfites can react with chlorophosphazenes to make fluorophosphazenes:
- (NPCl2)n + 2n KSO2F → (NPF2)n + 2n KCl + 2nSO2 n=3 or 4
Related
Fluorosulfite is in the category of halosulfite ions which include chlorosulfite, bromosulfite and iodosulfite. Related ions include cyanosulfite SO2CN.
List
| name | formula | weight | system | space group | unit cell | volume | density | properties | ref |
|---|---|---|---|---|---|---|---|---|---|
| NO | |||||||||
| 2-chloro-1,3-diisopropyl-4,5-dimethylimidazolium fluorosulfite | C11H20FClN2O2S | ||||||||
| 2-fluoro-1,3-diisopropyl-4,5-dimethylimidazolium fluorosulfite | C11H20F2N2O2S | P21/c | a=15.097 b=13.406 c=15.776 β=113.54 Z=8 | 2927 | |||||
| (difluoromethyl)triphenylphosphonium fluorosulfite | white, melt ~240 °C | ||||||||
| tetramethylammonium fluorosulfite | 157.21 | orthorhombic | Pbca | a=11.520 b=11.505 c=11.627 Z=8 | 1541.0 | 1.355 | |||
| tris-dimethylamino sulfonium fluorosulfite | 247.35 | orthorhombic | Pnma | a=14.690 b=11.174 c=7.3340 Z=4 | 1203.8 | 1.365 | |||
| tris-dimethylamino sulfoxonium fluorosulfite | 263.65 | orthorhombic | Pna21 | a=21.850 b=6.733 c=8.194 Z=4 | 1205.5 | 1.451 | |||
| potassium fluorosulfite | KSO2F | 122.16 | monoclinic | P21/m | a=6.9725 b=5.6713 c=4.6653 β=107.702° Z=2 | 175.75 | 2.308 | ||
| rubidium fluorosulfite | RbSO2F | monoclinic | P21/m | a=7.175 b=5.859 c=4.8416 β=107.18° Z=2 | 194.45 | 2.878 | |||
| alpha caesium fluorosulfite | α-CsSO2F | orthorhombic | Pnma | a=7.9098 b=6.6607 c=7.9893 z=4 | 420.91 | 3.408 | |||
| beta caesium fluorosulfite | β-CsSO2F | rhombohedral | R3m | a=6.5922 c=8.0050 z=3 | 301.27 | 3.571 | |||
References
- ^ Seel, F.; Meier, H. (December 1953). "Die Chemie des Nitrosyl-Ions. IX. Über den Chemismus des Bleikammerverfahrens". Zeitschrift für anorganische und allgemeine Chemie (in German). 274 (4–5): 197–222. doi:10.1002/zaac.19532740404. ISSN 0044-2313.
- Baasner, Bernd (2014). Houben-Weyl Methods of Organic Chemistry Vol. E 10a, 4th Edition Supplement: Organo-Fluorine Compounds - Fluorinating Agents and Their Application in Organic Synthesis. Georg Thieme Verlag. pp. 332–334. ISBN 978-3-13-181544-6.
- ^ Lork, Enno; Mews, Rüdiger; Viets, Detlef; Watson, Paul G.; Borrmann, Tobias; Vij, Ashwani; Boatz, Jerry A.; Christe, Karl O. (March 2001). "Structure of the SO 2 F - Anion, a Problem Case 1". Inorganic Chemistry. 40 (6): 1303–1311. doi:10.1021/ic000616p. ISSN 0020-1669. PMID 11300833.
- ^ Kessler, Ulrich; van Wüllen, Leo; Jansen, Martin (2001-12-01). "Structure of the Fluorosulfite Anion: Rotational Disorder of SO2F in the Alkali Metal Fluorosulfites and Crystal Structures of α- and β-CsSO2F". Inorganic Chemistry. 40 (27): 7040–7046. doi:10.1021/ic010303+. ISSN 0020-1669. PMID 11754288.
- Maulitz, Andreas H.; Boese, Roland; Kuhn, Norbert (April 1995). "Ab initio studies on halosulfite ions". Journal of Molecular Structure: THEOCHEM. 333 (3): 227–232. doi:10.1016/0166-1280(94)03955-K.
- Allcock, H. (2012). Phosphorus-Nitrogen Compounds: Cyclic, Linear, and High Polymeric Systems. Elsevier. p. 207. ISBN 978-0-323-14751-4.
- Robertson, Katherine N.; Land, Michael A.; Murphy, Luke J.; Doyle, Kirstin A.; Clyburne, Jason A. C. (2018-07-20). "Sulfur dioxide–halide ion complexes: a crystallographic investigation of bonding". Acta Crystallographica Section A. 74 (a1): a341. doi:10.1107/S0108767318096599. ISSN 2053-2733.
- Kornath, Andreas; Blecher, Oliver; Ludwig, Ralf (April 1999). "Synthesis and Characterization of Tetramethylammonium Cyanosulfite, (CH3)4NSO2CN". Journal of the American Chemical Society. 121 (16): 4019–4022. doi:10.1021/ja9833422. ISSN 0002-7863.
- ^ Kuhn, Norbert; Bohnen, Hans; Fahl, Joanna; Bläser, Dieter; Boese, Roland (December 1996). "Derivate des Imidazols, XIX. Koordination oder Reduktion? Zur Reaktion von 1,3-Diisopropyl-4,5-dimethylimidazol-2-yliden mit Schwefelhalogeniden und Schwefeloxidhalogeniden". Chemische Berichte (in German). 129 (12): 1579–1586. doi:10.1002/cber.19961291228.
- Zhu, Shi-Zheng; Huang, Qi-Chen; Wu, Kuang (September 1994). "Synthesis and Structure of (Difluoromethyl)triphenylphosphonium Fluorosulfite, Evidence for Formation of Difluorosulfene as an Intermediate". Inorganic Chemistry. 33 (20): 4584–4585. doi:10.1021/ic00098a028. ISSN 0020-1669.
| Compounds containing the sulfite group (SO2−3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||